Safety and Immunogenicity of a Tetravalent Dengue DNA Vaccine Administered with a Cationic Lipid-Based Adjuvant in a Phase 1 Clinical Trial
- PMID: 29363446
- PMCID: PMC5930886
- DOI: 10.4269/ajtmh.17-0416
Safety and Immunogenicity of a Tetravalent Dengue DNA Vaccine Administered with a Cationic Lipid-Based Adjuvant in a Phase 1 Clinical Trial
Abstract
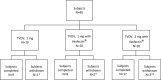
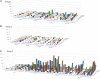
We conducted an open label, dose escalation Phase 1 clinical trial of a tetravalent dengue DNA vaccine (TVDV) formulated in Vaxfectin® to assess safety and immunogenicity. A total of 40 dengue- and flavivirus-naive volunteers received either low-dose (1 mg) TVDV alone (N = 10, group 1), low-dose TVDV (1 mg) formulated in Vaxfectin (N = 10, group 2), or high-dose TVDV (2 mg, group 3) formulated in Vaxfectin® (N = 20). Subjects were immunized intramuscularly with three doses on a 0-, 30-, 90-day schedule and monitored. Blood samples were obtained after each immunization and various time points thereafter to assess anti-dengue antibody and interferon gamma (IFNγ) T-cell immune responses. The most common adverse events (AEs) across all groups included mild to moderate pain and tenderness at the injection site, which typically resolved within 7 days. Common solicited signs and symptoms included fatigue (42.5%), headache (45%), and myalgias (47.5%). There were no serious AEs related to the vaccine or study procedures. No anti-dengue antibody responses were detected in group 1 subjects who received all three immunizations. There were minimal enzyme-linked immunosorbent assay and neutralizing antibody responses among groups 2 and 3 subjects who completed the immunization schedule. By contrast, IFNγ T-cell responses, regardless of serotype specificity, occurred in 70%, 50%, and 79% of subjects in groups 1, 2, and 3, respectively. The largest IFNγ T-cell responses were among group 3 subjects. We conclude that TVDV was safe and well-tolerated and elicited predominately anti-dengue T-cell IFNγ responses in a dose-related fashion.
Trial registration: ClinicalTrials.gov NCT00290147.
Figures
References
-
- Beckett CG, et al. 2011. Evaluation of a prototype dengue-1 DNA vaccine in a phase 1 clinical trial. Vaccine 29: 960–968. - PubMed
-
- Hartikka J, et al. 2001. Vaxfectin enhances the humoral immune response to plasmid DNA-encoded antigens. Vaccine 19: 1911–1923. - PubMed
-
- Hartikka J, Bozoukova V, Yang CK, Ye M, Rusalov D, Shlapobersky M, Vilalta A, Wei Q, Rolland A, Smith LR, 2009. Vaxfectin, a cationic lipid-based adjuvant for protein-based influenza vaccines. Vaccine 27: 6399–6403. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

