Nitric Oxide Production by Myeloid-Derived Suppressor Cells Plays a Role in Impairing Fc Receptor-Mediated Natural Killer Cell Function
- PMID: 29363526
- PMCID: PMC7184799
- DOI: 10.1158/1078-0432.CCR-17-0691
Nitric Oxide Production by Myeloid-Derived Suppressor Cells Plays a Role in Impairing Fc Receptor-Mediated Natural Killer Cell Function
Abstract
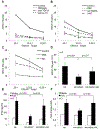
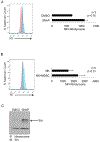
Purpose: mAbs are used to treat solid and hematologic malignancies and work in part through Fc receptors (FcRs) on natural killer cells (NK). However, FcR-mediated functions of NK cells from patients with cancer are significantly impaired. Identifying the mechanisms of this dysfunction and impaired response to mAb therapy could lead to combination therapies and enhance mAb therapy.Experimental Design: Cocultures of autologous NK cells and MDSC from patients with cancer were used to study the effect of myeloid-derived suppressor cells (MDSCs) on NK-cell FcR-mediated functions including antibody-dependent cellular cytotoxicity, cytokine production, and signal transduction in vitro Mouse breast cancer models were utilized to study the effect of MDSCs on antibody therapy in vivo and test the efficacy of combination therapies including a mAb and an MDSC-targeting agent.Results: MDSCs from patients with cancer were found to significantly inhibit NK-cell FcR-mediated functions including antibody-dependent cellular cytotoxicity, cytokine production, and signal transduction in a contact-independent manner. In addition, adoptive transfer of MDSCs abolished the efficacy of mAb therapy in a mouse model of pancreatic cancer. Inhibition of iNOS restored NK-cell functions and signal transduction. Finally, nonspecific elimination of MDSCs or inhibition of iNOS in vivo significantly improved the efficacy of mAb therapy in a mouse model of breast cancer.Conclusions: MDSCs antagonize NK-cell FcR-mediated function and signal transduction leading to impaired response to mAb therapy in part through nitric oxide production. Thus, elimination of MDSCs or inhibition of nitric oxide production offers a strategy to improve mAb therapy. Clin Cancer Res; 24(8); 1891-904. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Conflict of interest
Authors declare no conflict of interest.
Figures




Similar articles
-
Targeting the crosstalk between cytokine-induced killer cells and myeloid-derived suppressor cells in hepatocellular carcinoma.J Hepatol. 2019 Mar;70(3):449-457. doi: 10.1016/j.jhep.2018.10.040. Epub 2018 Nov 9. J Hepatol. 2019. PMID: 30414862 Free PMC article.
-
Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity.Clin Cancer Res. 2014 Aug 1;20(15):4096-106. doi: 10.1158/1078-0432.CCR-14-0635. Epub 2014 Jun 6. Clin Cancer Res. 2014. PMID: 24907113
-
NK Cell-Mediated Antitumor Effects of a Folate-Conjugated Immunoglobulin Are Enhanced by Cytokines.Cancer Immunol Res. 2016 Apr;4(4):323-336. doi: 10.1158/2326-6066.CIR-15-0168. Epub 2016 Feb 10. Cancer Immunol Res. 2016. PMID: 26865456 Free PMC article.
-
Polymorphonuclear Myeloid-Derived Suppressor Cells Are Abundant in Peripheral Blood of Cancer Patients and Suppress Natural Killer Cell Anti-Tumor Activity.Front Immunol. 2022 Jan 18;12:803014. doi: 10.3389/fimmu.2021.803014. eCollection 2021. Front Immunol. 2022. PMID: 35116033 Free PMC article. Review.
-
Natural Killer Cell Interactions With Myeloid Derived Suppressor Cells in the Tumor Microenvironment and Implications for Cancer Immunotherapy.Front Immunol. 2021 May 5;12:633205. doi: 10.3389/fimmu.2021.633205. eCollection 2021. Front Immunol. 2021. PMID: 34025641 Free PMC article. Review.
Cited by
-
Targeting Myeloid-Derived Suppressor Cells via Dual-Antibody Fluorescent Nanodiamond Conjugate.Nanomaterials (Basel). 2024 Sep 17;14(18):1509. doi: 10.3390/nano14181509. Nanomaterials (Basel). 2024. PMID: 39330666 Free PMC article.
-
Characterising how a single bout of exercise in people with myeloma affects clonal plasma cell and immune effector cell frequency in blood, and daratumumab efficacy in vitro.Brain Behav Immun Health. 2024 Sep 19;42:100865. doi: 10.1016/j.bbih.2024.100865. eCollection 2024 Dec. Brain Behav Immun Health. 2024. PMID: 39411424 Free PMC article.
-
Colorectal Liver Metastasis: Can Cytokines Make the Difference?Cancers (Basel). 2023 Nov 10;15(22):5359. doi: 10.3390/cancers15225359. Cancers (Basel). 2023. PMID: 38001618 Free PMC article. Review.
-
Metabolism in tumor microenvironment: Implications for cancer immunotherapy.MedComm (2020). 2020 Jun 3;1(1):47-68. doi: 10.1002/mco2.6. eCollection 2020 Jun. MedComm (2020). 2020. PMID: 34766109 Free PMC article. Review.
-
Importance of T, NK, CAR T and CAR NK Cell Metabolic Fitness for Effective Anti-Cancer Therapy: A Continuous Learning Process Allowing the Optimization of T, NK and CAR-Based Anti-Cancer Therapies.Cancers (Basel). 2021 Dec 30;14(1):183. doi: 10.3390/cancers14010183. Cancers (Basel). 2021. PMID: 35008348 Free PMC article. Review.
References
-
- Roda JM, Parihar R, Magro C, Nuovo GJ, Tridandapani S, Carson WE, 3rd. Natural killer cells produce T cell-recruiting chemokines in response to antibody-coated tumor cells. Cancer research 2006;66:517–26 - PubMed
-
- Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nature reviews Cancer 2012;12:278–87 - PubMed
-
- Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer 2000;88:577–83 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

