Tumor-Stroma IL1β-IRAK4 Feedforward Circuitry Drives Tumor Fibrosis, Chemoresistance, and Poor Prognosis in Pancreatic Cancer
- PMID: 29363544
- PMCID: PMC5890818
- DOI: 10.1158/0008-5472.CAN-17-1366
Tumor-Stroma IL1β-IRAK4 Feedforward Circuitry Drives Tumor Fibrosis, Chemoresistance, and Poor Prognosis in Pancreatic Cancer
Abstract
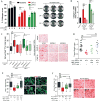
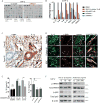
Targeting the desmoplastic stroma of pancreatic ductal adenocarcinoma (PDAC) holds promise to augment the effect of chemotherapy, but success in the clinic has thus far been limited. Preclinical mouse models suggest that near-depletion of cancer-associated fibroblasts (CAF) carries a risk of accelerating PDAC progression, underscoring the need to concurrently target key signaling mechanisms that drive the malignant attributes of both CAF and PDAC cells. We previously reported that inhibition of IL1 receptor-associated kinase 4 (IRAK4) suppresses NFκB activity and promotes response to chemotherapy in PDAC cells. In this study, we report that CAF in PDAC tumors robustly express activated IRAK4 and NFκB. IRAK4 expression in CAF promoted NFκB activity, drove tumor fibrosis, and supported PDAC cell proliferation, survival, and chemoresistance. Cytokine array analysis of CAF and microarray analysis of PDAC cells identified IL1β as a key cytokine that activated IRAK4 in CAF. Targeting IRAK4 or IL1β rendered PDAC tumors less fibrotic and more sensitive to gemcitabine. In clinical specimens of human PDAC, high stromal IL1β expression associated strongly with poor overall survival. Together, our studies establish a tumor-stroma IL1β-IRAK4 feedforward signal that can be therapeutically disrupted to increase chemotherapeutic efficacy in PDAC.Significance: Targeting the IL1β-IRAK4 signaling pathway potentiates the effect of chemotherapy in pancreatic cancer. Cancer Res; 78(7); 1700-12. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
The authors declare no potential conflicts of interest
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

