Fc-Mediated Anomalous Biodistribution of Therapeutic Antibodies in Immunodeficient Mouse Models
- PMID: 29363548
- PMCID: PMC5882577
- DOI: 10.1158/0008-5472.CAN-17-1958
Fc-Mediated Anomalous Biodistribution of Therapeutic Antibodies in Immunodeficient Mouse Models
Abstract
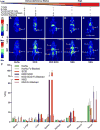
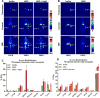
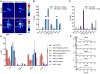
A critical benchmark in the development of antibody-based therapeutics is demonstration of efficacy in preclinical mouse models of human disease, many of which rely on immunodeficient mice. However, relatively little is known about how the biology of various immunodeficient strains impacts the in vivo fate of these drugs. Here we used immunoPET radiotracers prepared from humanized, chimeric, and murine mAbs against four therapeutic oncologic targets to interrogate their biodistribution in four different strains of immunodeficient mice bearing lung, prostate, and ovarian cancer xenografts. The immunodeficiency status of the mouse host as well as both the biological origin and glycosylation of the antibody contributed significantly to the anomalous biodistribution of therapeutic monoclonal antibodies in an Fc receptor-dependent manner. These findings may have important implications for the preclinical evaluation of Fc-containing therapeutics and highlight a clear need for biodistribution studies in the early stages of antibody drug development.Significance: Fc/FcγR-mediated immunobiology of the experimental host is a key determinant to preclinical in vivo tumor targeting and efficacy of therapeutic antibodies. Cancer Res; 78(7); 1820-32. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

