The arrestin-1 finger loop interacts with two distinct conformations of active rhodopsin
- PMID: 29363577
- PMCID: PMC5868258
- DOI: 10.1074/jbc.M117.817890
The arrestin-1 finger loop interacts with two distinct conformations of active rhodopsin
Abstract
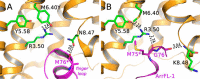
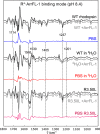
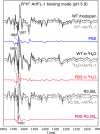
Signaling of the prototypical G protein-coupled receptor (GPCR) rhodopsin through its cognate G protein transducin (Gt) is quenched when arrestin binds to the activated receptor. Although the overall architecture of the rhodopsin/arrestin complex is known, many questions regarding its specificity remain unresolved. Here, using FTIR difference spectroscopy and a dual pH/peptide titration assay, we show that rhodopsin maintains certain flexibility upon binding the "finger loop" of visual arrestin (prepared as synthetic peptide ArrFL-1). We found that two distinct complexes can be stabilized depending on the protonation state of E3.49 in the conserved (D)ERY motif. Both complexes exhibit different interaction modes and affinities of ArrFL-1 binding. The plasticity of the receptor within the rhodopsin/ArrFL-1 complex stands in contrast to the complex with the C terminus of the Gt α-subunit (GαCT), which stabilizes only one specific substate out of the conformational ensemble. However, Gt α-subunit binding and both ArrFL-1-binding modes involve a direct interaction to conserved R3.50, as determined by site-directed mutagenesis. Our findings highlight the importance of receptor conformational flexibility and cytoplasmic proton uptake for modulation of rhodopsin signaling and thereby extend the picture provided by crystal structures of the rhodopsin/arrestin and rhodopsin/ArrFL-1 complexes. Furthermore, the two binding modes of ArrFL-1 identified here involve motifs of conserved amino acids, which indicates that our results may have elucidated a common modulation mechanism of class A GPCR-G protein/-arrestin signaling.
Keywords: Fourier transform IR (FTIR); G protein; G protein-coupled receptor (GPCR); arrestin; biased signaling; functional selectivity; rhodopsin.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
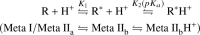

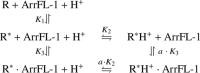
References
-
- Zhou X. E., He Y., de Waal P. W., Gao X., Kang Y., Van Eps N., Yin Y., Pal K., Goswami D., White T. A., Barty A., Latorraca N. R., Chapman H. N., Hubbell W. L., Dror R. O., Stevens R. C., Cherezov V., Gurevich V. V., Griffin P. R., Ernst O. P., Melcher K., and Xu H. E. (2017) Identification of phosphorylation codes for arrestin recruitment by G protein-coupled receptors. Cell 170, 457–469.e13 10.1016/j.cell.2017.07.002 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

