Deficient Surveillance and Phagocytic Activity of Myeloid Cells Within Demyelinated Lesions in Aging Mice Visualized by Ex Vivo Live Multiphoton Imaging
- PMID: 29363580
- PMCID: PMC6705888
- DOI: 10.1523/JNEUROSCI.2341-17.2018
Deficient Surveillance and Phagocytic Activity of Myeloid Cells Within Demyelinated Lesions in Aging Mice Visualized by Ex Vivo Live Multiphoton Imaging
Abstract
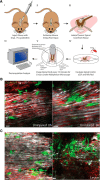
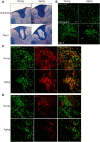
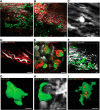
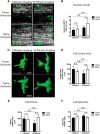
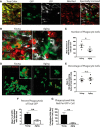
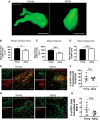
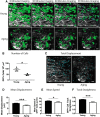
Aging impairs regenerative processes including remyelination, the synthesis of a new myelin sheath. Microglia and other infiltrating myeloid cells such as macrophages are essential for remyelination through mechanisms that include the clearance of inhibitory molecules within the lesion. Prior studies have shown that the quantity of myeloid cells and the clearance of inhibitory myelin debris are deficient in aging, contributing to the decline in remyelination efficiency with senescence. It is unknown, however, whether the impaired clearance of debris is simply the result of the reduced number of phagocytes or if the dynamic activity of myeloid cells within the demyelinating plaque also declines with aging and this question is relevant to the proper design of therapeutics to mobilize myeloid cells for repair. Herein, we describe a high-resolution multiphoton ex vivo live imaging protocol that visualizes individual myelinated/demyelinated axons and lipid-containing myeloid cells to investigate the demyelinated lesion of aging female mice. We found that aging lesions have fewer myeloid cells and that these have reduced phagocytosis of myelin. Although the myeloid cells are actively migratory within the lesion of young mice and have protrusions that seem to survey the environment, this motility and surveillance is significantly reduced in aging mice. Our results emphasize the necessity of not only increasing the number of phagocytes, but also enhancing their activity once they are within demyelinated lesions. The high-resolution live imaging of demyelinated lesions can serve as a platform with which to discover pharmacological agents that rejuvenate intralesional remodeling that promotes the repair of plaques.SIGNIFICANCE STATEMENT The repair of myelin after injury depends on myeloid cells that clear debris and release growth factors. As organisms age, remyelination becomes less efficient correspondent with fewer myeloid cells that populate the lesions. It is unknown whether the dynamic activity of cells within lesions is also altered with age. Herein, using high-resolution multiphoton ex vivo live imaging with several novel features, we report that myeloid cells within demyelinated lesions of aging mice have reduced motility, surveillance, and phagocytic activity, suggesting an intralesional impairment that may contribute to the age-related decline in remyelination efficiency. Medications to stimulate deficient aging myeloid cells should not only increase their representation, but also enter into lesions to stimulate their activity.
Keywords: demyelination; live imaging; macrophages; microglia.
Copyright © 2018 the authors 0270-6474/18/381973-16$15.00/0.
Figures
References
-
- Arnaud E, Zenker J, de Preux Charles AS, Stendel C, Roos A, Médard JJ, Tricaud N, Kleine H, Luscher B, Weis J, Suter U, Senderek J, Chrast R (2009) SH3TC2/KIAA1985 protein is required for proper myelination and the integrity of the node of ranvier in the peripheral nervous system. Proc Natl Acad Sci U S A 106:17528–17533. 10.1073/pnas.0905523106 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
