Neurogenesis during Abstinence Is Necessary for Context-Driven Methamphetamine-Related Memory
- PMID: 29363584
- PMCID: PMC5824740
- DOI: 10.1523/JNEUROSCI.2011-17.2018
Neurogenesis during Abstinence Is Necessary for Context-Driven Methamphetamine-Related Memory
Erratum in
-
Erratum: Galinato et al., "Neurogenesis during Abstinence Is Necessary for Context-Driven Methamphetamine-Related Memory".J Neurosci. 2019 Oct 2;39(40):7992. doi: 10.1523/JNEUROSCI.1959-19.2019. Epub 2019 Sep 11. J Neurosci. 2019. PMID: 31511431 Free PMC article. No abstract available.
Abstract
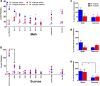
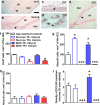
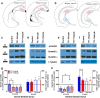
Abstinence from methamphetamine addiction enhances proliferation and differentiation of neural progenitors and increases adult neurogenesis in the dentate gyrus (DG). We hypothesized that neurogenesis during abstinence contributes to context-driven drug-seeking behaviors. To test this hypothesis, the pharmacogenetic rat model (GFAP-TK rats) was used to conditionally and specifically ablate neurogenesis in the DG. Male GFAP-TK rats were trained to self-administer methamphetamine or sucrose and were administered the antiviral drug valganciclovir (Valcyte) to produce apoptosis of actively dividing GFAP type 1 stem-like cells to inhibit neurogenesis during abstinence. Hippocampus tissue was stained for Ki-67, NeuroD, and DCX to measure levels of neural progenitors and immature neurons, and was stained for synaptoporin to determine alterations in mossy fiber tracts. DG-enriched tissue punches were probed for CaMKII to measure alterations in plasticity-related proteins. Whole-cell patch-clamp recordings were performed in acute brain slices from methamphetamine naive (controls) and methamphetamine experienced animals (+/-Valcyte). Spontaneous EPSCs and intrinsic excitability were recorded from granule cell neurons (GCNs). Reinstatement of methamphetamine seeking enhanced autophosphorylation of CaMKII, reduced mossy fiber density, and induced hyperexcitability of GCNs. Inhibition of neurogenesis during abstinence prevented context-driven methamphetamine seeking, and these effects correlated with reduced autophosphorylation of CaMKII, increased mossy fiber density, and reduced the excitability of GCNs. Context-driven sucrose seeking was unaffected. Together, the loss-of-neurogenesis data demonstrate that neurogenesis during abstinence assists with methamphetamine context-driven memory in rats, and that neurogenesis during abstinence is essential for the expression of synaptic proteins and plasticity promoting context-driven drug memory.SIGNIFICANCE STATEMENT Our work uncovers a mechanistic relationship between neurogenesis in the dentate gyrus and drug seeking. We report that the suppression of excessive neurogenesis during abstinence from methamphetamine addiction by a confirmed phamacogenetic approach blocked context-driven methamphetamine reinstatement and prevented maladaptive changes in expression and activation of synaptic proteins and basal synaptic function associated with learning and memory in the dentate gyrus. Our study is the first to demonstrate an interesting and dysfunctional role of adult hippocampal neurogenesis during abstinence to drug-seeking behavior in animals self-administering escalating amounts of methamphetamine. Together, these results support a direct role for the importance of adult neurogenesis during abstinence in compulsive-like drug reinstatement.
Keywords: CaMKII; NeuroD; electrophysiology; methamphetamine; self-administration; synaptoporin.
Copyright © 2018 the authors 0270-6474/18/382029-14$15.00/0.
Figures



Similar articles
-
The role of hippocampal adult neurogenesis in methamphetamine addiction.Brain Plast. 2018 Aug 10;3(2):157-168. doi: 10.3233/BPL-170058. Brain Plast. 2018. PMID: 30151340 Free PMC article. Review.
-
Neuroadaptations in the dentate gyrus following contextual cued reinstatement of methamphetamine seeking.Brain Struct Funct. 2018 Jun;223(5):2197-2211. doi: 10.1007/s00429-018-1615-3. Epub 2018 Feb 13. Brain Struct Funct. 2018. PMID: 29441405 Free PMC article.
-
A synthetic small-molecule Isoxazole-9 protects against methamphetamine relapse.Mol Psychiatry. 2018 Mar;23(3):629-638. doi: 10.1038/mp.2017.46. Epub 2017 Mar 28. Mol Psychiatry. 2018. PMID: 28348387 Free PMC article.
-
Sex Differences in Context-Driven Reinstatement of Methamphetamine Seeking is Associated with Distinct Neuroadaptations in the Dentate Gyrus.Brain Sci. 2018 Nov 28;8(12):208. doi: 10.3390/brainsci8120208. Brain Sci. 2018. PMID: 30487415 Free PMC article.
-
Neural mechanisms underlying incubation of methamphetamine craving: A mini-review.Pharmacol Biochem Behav. 2020 Dec;199:173058. doi: 10.1016/j.pbb.2020.173058. Epub 2020 Oct 23. Pharmacol Biochem Behav. 2020. PMID: 33250444 Free PMC article. Review.
Cited by
-
Functional neurogenesis over the years.Behav Brain Res. 2020 Mar 16;382:112470. doi: 10.1016/j.bbr.2020.112470. Epub 2020 Jan 7. Behav Brain Res. 2020. PMID: 31917241 Free PMC article. Review.
-
Chronic ethanol exposure differentially alters neuronal function in the medial prefrontal cortex and dentate gyrus.Neuropharmacology. 2021 Mar 1;185:108438. doi: 10.1016/j.neuropharm.2020.108438. Epub 2020 Dec 15. Neuropharmacology. 2021. PMID: 33333103 Free PMC article.
-
The role of hippocampal adult neurogenesis in methamphetamine addiction.Brain Plast. 2018 Aug 10;3(2):157-168. doi: 10.3233/BPL-170058. Brain Plast. 2018. PMID: 30151340 Free PMC article. Review.
-
Regulation of Adult Neurogenesis by Non-coding RNAs: Implications for Substance Use Disorders.Front Neurosci. 2018 Nov 22;12:849. doi: 10.3389/fnins.2018.00849. eCollection 2018. Front Neurosci. 2018. PMID: 30524229 Free PMC article. Review.
-
Isoxazole-9 reduces enhanced fear responses and retrieval in ethanol-dependent male rats.J Neurosci Res. 2021 Nov;99(11):3047-3065. doi: 10.1002/jnr.24932. Epub 2021 Sep 8. J Neurosci Res. 2021. PMID: 34496069 Free PMC article.
References
-
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, Wheeler AL, Guskjolen A, Niibori Y, Shoji H, Ohira K, Richards BA, Miyakawa T, Josselyn SA, Frankland PW (2014) Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 344:598–602. 10.1126/science.1248903 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
