The eardrums move when the eyes move: A multisensory effect on the mechanics of hearing
- PMID: 29363603
- PMCID: PMC5819440
- DOI: 10.1073/pnas.1717948115
The eardrums move when the eyes move: A multisensory effect on the mechanics of hearing
Abstract
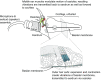
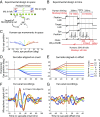
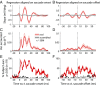
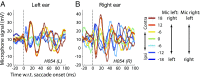
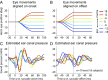
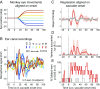
Interactions between sensory pathways such as the visual and auditory systems are known to occur in the brain, but where they first occur is uncertain. Here, we show a multimodal interaction evident at the eardrum. Ear canal microphone measurements in humans (n = 19 ears in 16 subjects) and monkeys (n = 5 ears in three subjects) performing a saccadic eye movement task to visual targets indicated that the eardrum moves in conjunction with the eye movement. The eardrum motion was oscillatory and began as early as 10 ms before saccade onset in humans or with saccade onset in monkeys. These eardrum movements, which we dub eye movement-related eardrum oscillations (EMREOs), occurred in the absence of a sound stimulus. The amplitude and phase of the EMREOs depended on the direction and horizontal amplitude of the saccade. They lasted throughout the saccade and well into subsequent periods of steady fixation. We discuss the possibility that the mechanisms underlying EMREOs create eye movement-related binaural cues that may aid the brain in evaluating the relationship between visual and auditory stimulus locations as the eyes move.
Keywords: EMREO; middle ear muscles; otoacoustic emissions; reference frame; saccade.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Groh JM, Sparks DL. Two models for transforming auditory signals from head-centered to eye-centered coordinates. Biol Cybern. 1992;67:291–302. - PubMed
-
- Groh JM, Trause AS, Underhill AM, Clark KR, Inati S. Eye position influences auditory responses in primate inferior colliculus. Neuron. 2001;29:509–518. - PubMed
-
- Porter KK, Metzger RR, Groh JM. Representation of eye position in primate inferior colliculus. J Neurophysiol. 2006;95:1826–1842. - PubMed
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

