Honokiol inhibits in vitro and in vivo growth of oral squamous cell carcinoma through induction of apoptosis, cell cycle arrest and autophagy
- PMID: 29363886
- PMCID: PMC5824386
- DOI: 10.1111/jcmm.13474
Honokiol inhibits in vitro and in vivo growth of oral squamous cell carcinoma through induction of apoptosis, cell cycle arrest and autophagy
Abstract
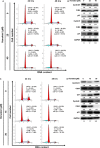
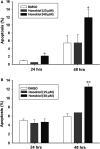
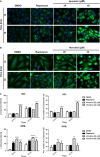
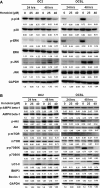
Honokiol, an active natural product derived from Magnolia officinalis, exerted anticancer effects through a variety of mechanisms on multiple types of cancers. In this study, the molecular mechanisms of honokiol in suppressing the human oral squamous cell carcinoma (OSCC) cells were evaluated. Treatment of two OSCC cell lines with honokiol resulted in reducing the cell proliferation and arresting the cell cycle at G1 stage which was correlated with the down-regulation of Cdk2 and Cdk4 and the up-regulation of cell cycle suppressors, p21 and p27. In addition, the caspase-dependent programmed cell death was substantially detected, and the autophagy was induced as the autophagosome formation and autophagic flux proceeded. Modulation of autophagy by autophagic inducer, rapamycin or inhibitors, 3-MA or bafilomycin, potentiated the honokiol-mediated anti-OSCC effects where honokiol exerted multiple actions in suppression of MAPK pathway and regulation of Akt/mTOR or AMPK pathways. As compared to clinical therapeutic agent, 5-FU, honokiol exhibited more potent activity against OSCC cells and synergistically enhanced the cytotoxic effect of 5-FU. Furthermore, orally administrated honokiol exerted effective antitumour activity in vivo in OSCC-xenografted mice. Thus, this study revealed that honokiol could be a promising candidate in preventing human OSCCs.
Keywords: Honokiol; apoptosis; autophagy; cell cycle arrest; human oral squamous cell carcinoma.
© 2018 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures






Similar articles
-
Honokiol, a chemopreventive agent against skin cancer, induces cell cycle arrest and apoptosis in human epidermoid A431 cells.Exp Biol Med (Maywood). 2011 Nov;236(11):1351-9. doi: 10.1258/ebm.2011.011030. Epub 2011 Sep 9. Exp Biol Med (Maywood). 2011. PMID: 21908486
-
Honokiol induces autophagic cell death in malignant glioma through reactive oxygen species-mediated regulation of the p53/PI3K/Akt/mTOR signaling pathway.Toxicol Appl Pharmacol. 2016 Aug 1;304:59-69. doi: 10.1016/j.taap.2016.05.018. Epub 2016 May 25. Toxicol Appl Pharmacol. 2016. PMID: 27236003
-
TEAD4-YAP interaction regulates tumoral growth by controlling cell-cycle arrest at the G1 phase.Biochem Biophys Res Commun. 2017 Apr 29;486(2):385-390. doi: 10.1016/j.bbrc.2017.03.050. Epub 2017 Mar 14. Biochem Biophys Res Commun. 2017. PMID: 28315328
-
PI3K/AKT Signaling Pathway Mediated Autophagy in Oral Carcinoma - A Comprehensive Review.Int J Med Sci. 2024 Apr 29;21(6):1165-1175. doi: 10.7150/ijms.94566. eCollection 2024. Int J Med Sci. 2024. PMID: 38774756 Free PMC article. Review.
-
Honokiol: a novel natural agent for cancer prevention and therapy.Curr Mol Med. 2012 Dec;12(10):1244-52. doi: 10.2174/156652412803833508. Curr Mol Med. 2012. PMID: 22834827 Free PMC article. Review.
Cited by
-
Evodiamine exerts anti-cancer activity including growth inhibition, cell cycle arrest, and apoptosis induction in human follicular thyroid cancers.Am J Cancer Res. 2024 Oct 25;14(10):4989-4999. doi: 10.62347/DNTG2917. eCollection 2024. Am J Cancer Res. 2024. PMID: 39553211 Free PMC article.
-
Smokeless tobacco extract inhibits proliferation and promotes apoptosis in oral mucous fibroblasts.Oncol Lett. 2018 Oct;16(4):5066-5074. doi: 10.3892/ol.2018.9252. Epub 2018 Aug 2. Oncol Lett. 2018. PMID: 30250574 Free PMC article.
-
Pharmacokinetic and Metabolic Profiling of Key Active Components of Dietary Supplement Magnolia officinalis Extract for Prevention against Oral Carcinoma.J Agric Food Chem. 2020 Jun 17;68(24):6576-6587. doi: 10.1021/acs.jafc.0c01475. Epub 2020 Jun 4. J Agric Food Chem. 2020. PMID: 32348135 Free PMC article.
-
Honokiol Is More Potent than Magnolol in Reducing Head and Neck Cancer Cell Growth.Curr Issues Mol Biol. 2024 Sep 25;46(10):10731-10744. doi: 10.3390/cimb46100637. Curr Issues Mol Biol. 2024. PMID: 39451517 Free PMC article.
-
Molecular mechanism of the anti-gastric cancer activity of 1,2,3,6-tetra-O-galloyl-β-D-glucose isolated from Trapa bispinosa Roxb. shell in vitro.PLoS One. 2022 Jun 2;17(6):e0269013. doi: 10.1371/journal.pone.0269013. eCollection 2022. PLoS One. 2022. PMID: 35653387 Free PMC article.
References
-
- Parkin DM, Bray F, Ferlay J, et al Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55: 74–108. - PubMed
-
- Sineviciand N, O'Sullivan J. Oral cancer: deregulated molecular events and their use as biomarkers. Oral Oncol. 2016; 61: 12–8. - PubMed
-
- Jemal A, Siegel R, Ward E, et al Cancer statistics, 2009. CA Cancer J Clin. 2009; 59: 225–49. - PubMed
-
- Jemal A, Bray F, Center MM, et al Global cancer statistics. CA Cancer J Clin. 2011; 61: 69–90. - PubMed
-
- Trivedy CR, Craig G, Warnakulasuriya S. The oral health consequences of chewing areca nut. Addict Biol. 2002; 7: 115–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

