Developments in Glycopeptide Antibiotics
- PMID: 29363950
- PMCID: PMC5952257
- DOI: 10.1021/acsinfecdis.7b00258
Developments in Glycopeptide Antibiotics
Abstract
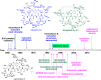
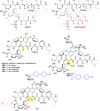
Glycopeptide antibiotics (GPAs) are a key weapon in the fight against drug resistant bacteria, with vancomycin still a mainstream therapy against serious Gram-positive infections more than 50 years after it was first introduced. New, more potent semisynthetic derivatives that have entered the clinic, such as dalbavancin and oritavancin, have superior pharmacokinetic and target engagement profiles that enable successful treatment of vancomycin-resistant infections. In the face of resistance development, with multidrug resistant (MDR) S. pneumoniae and methicillin-resistant Staphylococcus aureus (MRSA) together causing 20-fold more infections than all MDR Gram-negative infections combined, further improvements are desirable to ensure the Gram-positive armamentarium is adequately maintained for future generations. A range of modified glycopeptides has been generated in the past decade via total syntheses, semisynthetic modifications of natural products, or biological engineering. Several of these have undergone extensive characterization with demonstrated in vivo efficacy, good PK/PD profiles, and no reported preclinical toxicity; some may be suitable for formal preclinical development. The natural product monobactam, cephalosporin, and β-lactam antibiotics all spawned multiple generations of commercially and clinically successful semisynthetic derivatives. Similarly, next-generation glycopeptides are now technically well positioned to advance to the clinic, if sufficient funding and market support returns to antibiotic development.
Keywords: antibiotics; antimicrobial resistance; glycopeptides; vancomycin.
Conflict of interest statement
The authors declare the following competing financial interest(s): M.A.C. and M.A.T.B. are inventors on WO 2015/117196 A, describing new glycopeptide derivatives discussed in this Review and subject to commercialization activities.
Figures












References
-
- World Health Organization . (2017) Antibacterial agents in clinical development: An analysis of the antibacterial clinical development pipeline, including tuberculosis, WHO/EMP/IAU/2017.11, WHO, Geneva, http://apps.who.int/iris/bitstream/10665/258965/1/WHO-EMP-IAU-2017.11-en... (accessed 22 November 2017).
-
- Grayeff Y. (2014) Cubist Q4 revenues + 22%; Cubicin annual sales top $1B, https://seekingalpha.com/news/1503931-cubist-q4-revenues-plus-22-percent... (accessed 22 November 2017).
-
- CDC . (2013) Antibiotic Resistance Threats in the United States, 2013, U.S. Department of Health and Human Services (Centers for Disease Control and Prevention), Atlanta, http://www.cdc.gov/drugresistance/threat-report-2013 (accessed 22 November 2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

