Lycopene Attenuates Tulathromycin and Diclofenac Sodium-Induced Cardiotoxicity in Mice
- PMID: 29364179
- PMCID: PMC5855566
- DOI: 10.3390/ijms19020344
Lycopene Attenuates Tulathromycin and Diclofenac Sodium-Induced Cardiotoxicity in Mice
Abstract
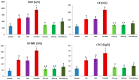
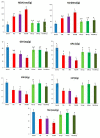
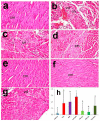
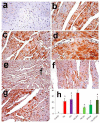
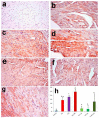
Recent experiments showed a potential cardiotoxic effect of the macrolide antibiotic (tulathromycin). This study was performed to investigate whether diclofenac sodium (DFS) potentiates the cardiotoxicity of tulathromycin and increases the cardioprotective effects of lycopene against DFS and tulathromycin. Seven groups (eight per group) of adult Swiss albino mice received saline (control), tulathromycin (a single subcutaneous dose of 28 mg/kg/bw on day 14), DFS (a single oral dose of 100 mg/kg/bw on day 14), tulathromycin plus DFS, or lycopene (oral, 10 mg/kg/bw daily for 15 d) combined with tulathromycin, DFS, or both. Compared to the control group, the administration of tulathromycin or DFS (individually or in combination) caused significantly elevated (p < 0.05) serum levels of Creatine kinase-myocardial B fraction (CK-MB), lactate dehydrogenase, and cardiac-specific troponin-T and tissue levels of nitric oxide and malondialdehyde that were accompanied by significantly decreased tissue reduced glutathione content and glutathione peroxidase, superoxide dismutase, and catalase antioxidant enzyme activity. Upon histopathological and immunohistochemical examination, the mean pathology scores and the percentages of caspase-3-, Bax-, and CK-positive regions were significantly higher in the tulathromycin- and/or DFS-treated groups than in control mice. For all these parameters, the pathological changes were more significant in the tulathromycin-DFS combination group than in mice treated with either drug individually. Interestingly, co-administration of lycopene with tulathromycin and/or DFS significantly ameliorated the changes described above. In conclusion, DFS could potentiate the cardiotoxic effects of tulathromycin, whereas lycopene can serve as a cardioprotective agent against DFS and tulathromycin.
Keywords: cardiotoxicity; diclofenac sodium; lycopene; mice; tulathromycin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ibrahim N., Burke J., Beattie D. The sensitivity of rat liver and yeast mitochondrial ribosomes to inhibitors of protein synthesis. J. Biol. Chem. 1974;249:6806–6811. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

