Engineered photoproteins that give rise to photosynthetically-incompetent bacteria are effective as photovoltaic materials for biohybrid photoelectrochemical cells
- PMID: 29364305
- PMCID: PMC5903125
- DOI: 10.1039/c7fd00190h
Engineered photoproteins that give rise to photosynthetically-incompetent bacteria are effective as photovoltaic materials for biohybrid photoelectrochemical cells
Abstract
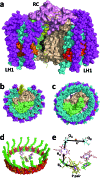
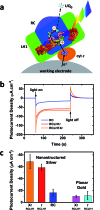
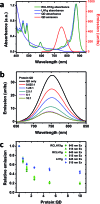
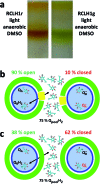
Reaction centre/light harvesting proteins such as the RCLH1X complex from Rhodobacter sphaeroides carry out highly quantum-efficient conversion of solar energy through ultrafast energy transfer and charge separation, and these pigment-proteins have been incorporated into biohybrid photoelectrochemical cells for a variety of applications. In this work we demonstrate that, despite not being able to support normal photosynthetic growth of Rhodobacter sphaeroides, an engineered variant of this RCLH1X complex lacking the PufX protein and with an enlarged light harvesting antenna is unimpaired in its capacity for photocurrent generation in two types of bio-photoelectrochemical cells. Removal of PufX also did not impair the ability of the RCLH1 complex to act as an acceptor of energy from synthetic light harvesting quantum dots. Unexpectedly, the removal of PufX led to a marked improvement in the overall stability of the RCLH1 complex under heat stress. We conclude that PufX-deficient RCLH1 complexes are fully functional in solar energy conversion in a device setting and that their enhanced structural stability could make them a preferred choice over their native PufX-containing counterpart. Our findings on the competence of RCLH1 complexes for light energy conversion in vitro are discussed with reference to the reason why these PufX-deficient proteins are not capable of light energy conversion in vivo.
Figures



References
-
- Badura A., Kothe T., Schuhmann W., Rogner M. Energy Environ. Sci. 2011;4:3263–3274.
-
- Boghossian A. A., Ham M.-H., Choi J. H., Strano M. S. Energy Environ. Sci. 2011;4:3834–3843.
-
- Wang F., Liu X., Willner I. Adv. Mater. 2013;25:349–377. - PubMed
-
- Kim Y., Shin S. A., Lee J., Yang K. D., Nam K. T. Nanotechnology. 2014;25:342001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

