Elevated androgen levels induce hyperinsulinemia through increase in Ins1 transcription in pancreatic beta cells in female rats
- PMID: 29365042
- PMCID: PMC6279097
- DOI: 10.1093/biolre/ioy017
Elevated androgen levels induce hyperinsulinemia through increase in Ins1 transcription in pancreatic beta cells in female rats
Abstract
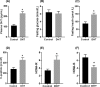
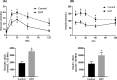
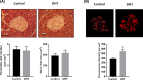
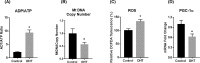
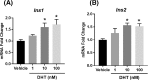
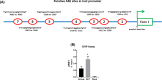
Hyperandrogenism is associated with hyperinsulinemia and insulin resistance in adult females. We tested whether androgens dysregulate pancreatic beta cell function to induce hyperinsulinemia through transcriptional regulation of insulin gene (Ins) in the islets. Adult female Wistar rats implanted with dihydrotestosterone (DHT; 7.5-mg, 90-d release) or placebo pellets were examined after 10 weeks. DHT exposure increased plasma DHT levels by 2-fold similar to that in polycystic ovary syndrome in women. DHT exposure induced hyperinsulinemia with increased HOMA-IR index in fasting state and glucose intolerance and exaggerated insulin responses following glucose tolerance test. DHT females had no change in islet number, size and beta cell proliferation/apoptosis but exhibited significant mitochondrial dysfunction (higher ADP/ATP ratio, decreased mtDNA copy number, increased reactive oxygen production and downregulation of mitochondrial biogenesis) and enhanced glucose-stimulated insulin secretion. Ins expression was increased in DHT islets. In vitro incubation of control islets with DHT dose dependently stimulated Ins transcription. Analysis of Ins1 gene revealed a putative androgen responsive element in the promoter. Chromatin-immunoprecipitation assays showed that androgen receptors bind to this element in response to DHT stimulation. Furthermore, reporter assays showed that the promoter element is highly responsive to androgens. Insulin-stimulated glucose uptake in skeletal muscle was decreased with associated decrease in IRβ expression in DHT females. Our studies identified a novel androgen-mediated mechanism for the control of Ins expression via transcriptional regulation providing a molecular mechanism linking elevated androgens and hyperinsulemia. Decreased IRβ expression in the skeletal muscles may contribute, in part, to glucose intolerance in this model.
Figures
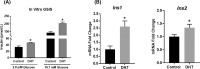


References
-
- Ciampelli M, Leoni F, Cucinelli F, Mancuso S, Panunzi S, De GA, Lanzone A. Assessment of insulin sensitivity from measurements in the fasting state and during an oral glucose tolerance test in polycystic ovary syndrome and menopausal patients. J. Clin. Endocrinol. Metab 2005; 90:1398–1406. - PubMed
-
- DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil. Steril 2005; 83:1454–1460. - PubMed
-
- Ovalle F, Azziz R. Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. Fertil. Steril 2002; 77:1095–1105. - PubMed
-
- Waterworth DM, Bennett ST, Gharani N, McCarthy MI, Hague S, Batty S, Conway GS, White D, Todd JA, Franks S, Williamson R. Linkage and association of insulin gene VNTR regulatory polymorphism with polycystic ovary syndrome. Lancet 1997; 349:986–990. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

