Microparticulate Caspase 1 Regulates Gasdermin D and Pulmonary Vascular Endothelial Cell Injury
- PMID: 29365280
- PMCID: PMC6039876
- DOI: 10.1165/rcmb.2017-0393OC
Microparticulate Caspase 1 Regulates Gasdermin D and Pulmonary Vascular Endothelial Cell Injury
Abstract
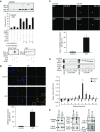
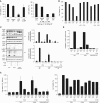
Lung endothelial cell apoptosis and injury occur throughout all stages of acute lung injury/acute respiratory distress syndrome and impact disease progression. Caspases 1, 4, and 5 are essential for completion of the apoptotic program known as pyroptosis that also involves proinflammatory cytokines. Because gasdermin D (GSDMD) mediates pyroptotic death and is essential for pore formation, we hypothesized that it might direct caspase 1-encapsulated microparticle (MP) release and mediate endothelial cell death. Our present work provides evidence that GSDMD is released by LPS-stimulated THP-1 monocytic cells, where it is packaged into microparticles together with active caspase 1. Furthermore, only MP released from stimulated monocytic cells that contain both cleaved GSDMD and active caspase 1 induce endothelial cell apoptosis. MPs pretreated with caspase 1 inhibitor Y-VAD or pan-caspase inhibitor Z-VAD do not contain cleaved GSDMD. MPs from caspase 1-knockout cells are also deficient in p30 active GSDMD, further confirming that caspase 1 regulates GSDMD function. Although control MPs contained cleaved GSDMD without caspase 1, these fractions were unable to induce cell death, suggesting that encapsulation of both caspase 1 and GSDMD is essential for cell death induction. Release of microparticulate active caspase 1 was abrogated in GSDMD knockout cells, although cytosolic caspase 1 activation was not impaired. Last, higher concentrations of microparticulate GSDMD were detected in the plasma of septic patients with acute respiratory distress syndrome than in that of healthy donors. Taken together, these findings suggest that GSDMD regulates the release of microparticulate active caspase 1 from monocytes essential for induction of cell death and thereby may play a critical role in sepsis-induced endothelial cell injury.
Figures


Similar articles
-
Mononuclear Phagocyte-Derived Microparticulate Caspase-1 Induces Pulmonary Vascular Endothelial Cell Injury.PLoS One. 2015 Dec 28;10(12):e0145607. doi: 10.1371/journal.pone.0145607. eCollection 2015. PLoS One. 2015. PMID: 26710067 Free PMC article.
-
Francisella induced microparticulate caspase-1/gasdermin-D activation is regulated by NLRP3 independent of Pyrin.PLoS One. 2018 Dec 31;13(12):e0209931. doi: 10.1371/journal.pone.0209931. eCollection 2018. PLoS One. 2018. PMID: 30596757 Free PMC article.
-
Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death.Nature. 2015 Oct 29;526(7575):660-5. doi: 10.1038/nature15514. Epub 2015 Sep 16. Nature. 2015. PMID: 26375003
-
Recent Advances in the Molecular Mechanisms Underlying Pyroptosis in Sepsis.Mediators Inflamm. 2018 Mar 7;2018:5823823. doi: 10.1155/2018/5823823. eCollection 2018. Mediators Inflamm. 2018. PMID: 29706799 Free PMC article. Review.
-
Gasdermins and their role in immunity and inflammation.J Exp Med. 2019 Nov 4;216(11):2453-2465. doi: 10.1084/jem.20190545. Epub 2019 Sep 23. J Exp Med. 2019. PMID: 31548300 Free PMC article. Review.
Cited by
-
Extracellular vesicles and lupus nephritis - New insights into pathophysiology and clinical implications.J Autoimmun. 2020 Dec;115:102540. doi: 10.1016/j.jaut.2020.102540. Epub 2020 Sep 4. J Autoimmun. 2020. PMID: 32893081 Free PMC article. Review.
-
Regulation of Mesenchymal Cell Fate by Transfer of Active Gasdermin-D via Monocyte-Derived Extracellular Vesicles.J Immunol. 2023 Mar 15;210(6):832-841. doi: 10.4049/jimmunol.2200511. J Immunol. 2023. PMID: 36688687 Free PMC article.
-
Emricasan for the treatment of liver cirrhosis: a meta-analysis of randomized controlled trials.Afr Health Sci. 2023 Jun;23(2):402-408. doi: 10.4314/ahs.v23i2.46. Afr Health Sci. 2023. PMID: 38223578 Free PMC article.
-
The role of pyroptosis in endothelial dysfunction induced by diseases.Front Immunol. 2023 Jan 9;13:1093985. doi: 10.3389/fimmu.2022.1093985. eCollection 2022. Front Immunol. 2023. PMID: 36776394 Free PMC article. Review.
-
Impact of extracellular vesicles on the pathogenesis, diagnosis, and potential therapy in cardiopulmonary disease.Front Pharmacol. 2023 Feb 20;14:1081015. doi: 10.3389/fphar.2023.1081015. eCollection 2023. Front Pharmacol. 2023. PMID: 36891265 Free PMC article. Review.
References
-
- Hugel B, Martínez MC, Kunzelmann C, Freyssinet JM. Membrane microparticles: two sides of the coin. Physiology (Bethesda) 2005;20:22–27. - PubMed
-
- Distler JH, Huber LC, Gay S, Distler O, Pisetsky DS. Microparticles as mediators of cellular cross-talk in inflammatory disease. Autoimmunity. 2006;39:683–690. - PubMed
-
- Martínez MC, Tesse A, Zobairi F, Andriantsitohaina R. Shed membrane microparticles from circulating and vascular cells in regulating vascular function. Am J Physiol Heart Circ Physiol. 2005;288:H1004–H1009. - PubMed
-
- Diamant M, Tushuizen ME, Sturk A, Nieuwland R. Cellular microparticles: new players in the field of vascular disease? Eur J Clin Invest. 2004;34:392–401. - PubMed
-
- Martin S, Tesse A, Hugel B, Martínez MC, Morel O, Freyssinet JM, et al. Shed membrane particles from T lymphocytes impair endothelial function and regulate endothelial protein expression. Circulation. 2004;109:1653–1659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

