The human IL-15 superagonist ALT-803 directs SIV-specific CD8+ T cells into B-cell follicles
- PMID: 29365313
- PMCID: PMC5787870
- DOI: 10.1182/bloodadvances.2017012971
The human IL-15 superagonist ALT-803 directs SIV-specific CD8+ T cells into B-cell follicles
Abstract
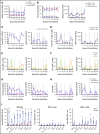
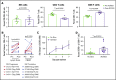
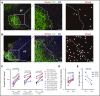
Sequestering of latent HIV in follicular helper T cells within B-cell follicles that largely exclude cytotoxic T cells is a major barrier to cellular immune-based approaches to eradicate HIV. Here, we show that the clinical-grade human interleukin-15 (IL-15) superagonist ALT-803 activates and redirects simian immunodeficiency virus (SIV)-specific CD8+ T cells from the peripheral blood into B-cell follicles. In agreement with the increased trafficking of SIV-specific cytotoxic T cells to sites of cryptic viral replication, lymph nodes of elite controlling macaques contained fewer cells expressing SIV RNA or harboring SIV DNA post-ALT-803 treatment. These data establish ALT-803 as an immunotherapeutic for HIV and other chronic viral pathogens that evade host immunity by persisting in B-cell follicles.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: E.K.J. and H.C.W. are employees of Altor Bioscience Corporation. The remaining authors declare no competing financial interests.
Figures


References
-
- Connick E, Mattila T, Folkvord JM, et al. . CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J Immunol. 2007;178(11):6975-6983. - PubMed
-
- Leong YA, Chen Y, Ong HS, et al. . CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol. 2016;17(10):1187-1196. - PubMed
-
- He R, Hou S, Liu C, et al. . Follicular CXCR5-expressing CD8(+) T cells curtail chronic viral infection. Nature. 2016;537(7620):412-428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

