Immune rebound associates with a favorable clinical response to autologous HSCT in systemic sclerosis patients
- PMID: 29365321
- PMCID: PMC5787873
- DOI: 10.1182/bloodadvances.2017011072
Immune rebound associates with a favorable clinical response to autologous HSCT in systemic sclerosis patients
Abstract
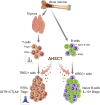
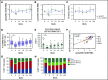
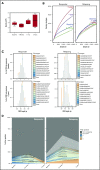
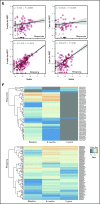
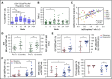
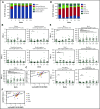
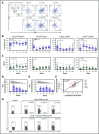
To evaluate the immunological mechanisms associated with clinical outcomes after autologous hematopoietic stem cell transplantation (AHSCT), focusing on regulatory T- (Treg) and B- (Breg) cell immune reconstitution, 31 systemic sclerosis (SSc) patients underwent simultaneous clinical and immunological evaluations over 36-month posttransplantation follow-up. Patients were retrospectively grouped into responders (n = 25) and nonresponders (n = 6), according to clinical response after AHSCT. Thymic function and B-cell neogenesis were respectively assessed by quantification of DNA excision circles generated during T- and B-cell receptor rearrangements. At the 1-year post-AHSCT evaluation of the total set of transplanted SSc patients, thymic rebound led to renewal of the immune system, with higher T-cell receptor (TCR) diversity, positive correlation between recent thymic emigrant and Treg counts, and higher expression of CTLA-4 and GITR on Tregs, when compared with pretransplant levels. In parallel, increased bone marrow output of newly generated naive B-cells, starting at 6 months after AHSCT, renovated the B-cell populations in peripheral blood. At 6 and 12 months after AHSCT, Bregs increased and produced higher interleukin-10 levels than before transplant. When the nonresponder patients were evaluated separately, Treg and Breg counts did not increase after AHSCT, and high TCR repertoire overlap between pre- and posttransplant periods indicated maintenance of underlying disease mechanisms. These data suggest that clinical improvement of SSc patients is related to increased counts of newly generated Tregs and Bregs after AHSCT as a result of coordinated thymic and bone marrow rebound.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


References
-
- Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685-1699. - PubMed
-
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360(19):1989-2003. - PubMed
-
- Nannini C, West CP, Erwin PJ, Matteson EL. Effects of cyclophosphamide on pulmonary function in patients with scleroderma and interstitial lung disease: a systematic review and meta-analysis of randomized controlled trials and observational prospective cohort studies. Arthritis Res Ther. 2008;10(5):R124. - PMC - PubMed
-
- Tashkin DP, Elashoff R, Clements PJ, et al. ; Scleroderma Lung Study Research Group. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655-2666. - PubMed
-
- Tashkin DP, Roth MD, Clements PJ, et al. ; Sclerodema Lung Study II Investigators. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4(9):708-719. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

