Treg-specific deletion of NKAP results in severe, systemic autoimmunity due to peripheral loss of Tregs
- PMID: 29366602
- PMCID: PMC6205721
- DOI: 10.1016/j.jaut.2017.12.013
Treg-specific deletion of NKAP results in severe, systemic autoimmunity due to peripheral loss of Tregs
Abstract
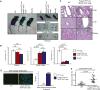
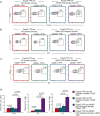
Regulatory T cells are critical for the generation and maintenance of peripheral tolerance. Conditional deletion of the transcriptional repressor NKAP in Tregs using Foxp3-YFP-cre NKAP conditional knockout mice causes aggressive autoimmunity characterized by thymic atrophy, lymphadenopathy, peripheral T cell activation, generation of autoantibodies, immune infiltration into several organs, and crusty skin at 3 weeks of age, similar to that of "scurfy" Foxp3-mutant mice. While Treg development in the thymus proceeds normally in the absence of NKAP, there is a severe loss of thymically-derived Tregs in the periphery. NKAP-deficient Tregs have a recent thymic emigrant phenotype, and are attacked by complement in a cell-intrinsic manner in the periphery. Previously, we demonstrated that NKAP is required for conventional T cell maturation as it prevents complement-mediated attack in the periphery. We now show that Tregs undergo a similar maturation process as conventional T cells, requiring NKAP to acquire complement resistance after thymic egress.
Keywords: Complement; NKAP; Scurfy; Tregs.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Jordan MS, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2001;2:301–306. - PubMed
-
- Hsieh C-S, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat. Immunol. 2006;7:401–410. - PubMed
-
- Hsieh C-SS, et al. Recognition of the Peripheral Self by Naturally Arising CD25+ CD4+ T Cell Receptors. Immunity. 2004;21:267–277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

