Enhanced Immunosuppressive Properties of Human Mesenchymal Stem Cells Primed by Interferon-γ
- PMID: 29366627
- PMCID: PMC5898027
- DOI: 10.1016/j.ebiom.2018.01.002
Enhanced Immunosuppressive Properties of Human Mesenchymal Stem Cells Primed by Interferon-γ
Abstract
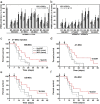
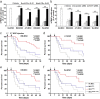
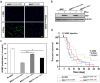
Mesenchymal stem cells (MSCs) are of particular interest for the treatment of immune-related diseases owing to their immunosuppressive properties. In this study, we aimed to identify the effect of interferon (IFN)-γ priming on immunomodulation by MSCs and elucidate the possible mechanism underlying their properties for the clinical treatment of allogeneic conflicts. Infusion of MSCs primed with IFN-γ significantly reduced the symptoms of graft-versus-host disease (GVHD) in NOD-SCID mice, thereby increasing survival rate when compared with naïve MSC-infused mice. However, infusion of IFN-γ-primed MSCs in which indoleamine 2,3-dioxygenase (IDO) was downregulated did not elicit this effect. The IDO gene was expressed in MSCs via the IFN-γ-Janus kinase (JAK)-signal transducer and activator of transcription 1 (STAT1) pathway, and the infusion of IDO-over-expressing MSCs increased survival rate in an in vivo GVHD model, similar to infusion of IFN-γ-primed MSCs. These data indicate that IFN-γ production by activated T-cells is correlated with the induction of IDO expression in MSCs via the IFN-γ-JAK-STAT1 pathway, which in turn results in the suppression of T-cell proliferation. Our findings also suggest that cell therapy based on MSCs primed with IFN-γ can be used for the clinical treatment of allogeneic conflicts, including GVHD.
Keywords: Cell therapy; Graft-versus-host disease; Indoleamine 2,3-dioxygenase; Interferon-γ; Mesenchymal stem cell.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Augello A., Tasso R., Negrini S.M., Amateis A., Indiveri F., Cancedda R., Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur. J. Immunol. 2005;35:1482–1490. - PubMed
-
- Barry F.P., Murphy J.M. Mesenchymal stem cells: clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 2004;36:568–584. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

