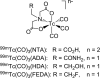
Re(CO)3([18F]FEDA), a novel 18F PET renal tracer: Radiosynthesis and preclinical evaluation
- PMID: 29367095
- PMCID: PMC5817033
- DOI: 10.1016/j.nucmedbio.2017.12.001
Re(CO)3([18F]FEDA), a novel 18F PET renal tracer: Radiosynthesis and preclinical evaluation
Abstract
Introduction: Our previous work demonstrated that the 99mTc renal tracer, 99mTc(CO)3(FEDA) (99mTc-1), has a rapid clearance comparable in rats to that of 131I-OIH, the radioactive gold standard for the measurement of effective renal plasma flow. The uncharged fluoroethyl pendant group of 99mTc-1 provides a route to the synthesis of a structurally analogous rhenium-tricarbonyl 18F renal imaging agent, Re(CO)3([18F]FEDA) (18F-1). Our goal was to develop an efficient one-step method for the preparation of 18F-1 and to compare its pharmacokinetic properties with those of 131I-OIH in rats.
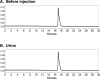
Methods: 18F-1 was prepared by the nucleophilic 18F-fluorination of its tosyl precursor. The labeled compound was isolated by HPLC and subsequently evaluated in Sprague-Dawley rats using 131I-OIH as an internal control and by dynamic PET/CT imaging. Plasma protein binding (PPB) and erythrocyte uptake (RCB) were determined and the urine was analyzed for metabolites.
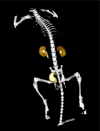
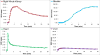
Results: 18F-1 was efficiently prepared as a single species with high radiochemical purity (>99%) and it displayed high radiochemical stability in vitro and in vivo. PPB was 87% and RCB was 21%. Biodistribution studies confirmed rapid renal extraction and high specificity for renal excretion, comparable to that of 131I-OIH, with minimal hepatic/gastrointestinal elimination. The activity in the urine, as a percentage of 131I-OIH, was 92% and 95% at 10 and 60 min, respectively. All other organs (heart, spleen, lungs) showed a negligible tracer uptake (<0.4% ID). Dynamic microPET/CT imaging demonstrated rapid transit of 18F-1 through the kidneys and into the bladder; there was no demonstrable activity in bone verifying the absence of free [18F]fluoride.
Conclusions: 18F-1 exhibited a high specificity for the kidney, rapid renal excretion comparable to that of 131I-OIH and high in vivo radiochemical stability. Not only is 18F-1 a promising PET renal tracer, but it provides a route to the development of a pair of analogous 18F/99mTc renal imaging agents with almost identical structures and comparable pharmacokinetic properties. These promising in vivo results warrant subsequent evaluation in humans.
Keywords: Biodistribution; PET imaging; Radiolabeling; Re(CO)(3)([(18)F]FEDA); Renal radiotracer.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




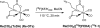
References
-
- Lane BR, Poggio ED, Herts BR, Novick AC, Campbell SC. Renal function assesment in the era of chronic kidney disease: renewed emphasis on renal function centered patient care. J Urol. 2009;182:435–44. - PubMed
-
- Prigent A. Monitoring renal function and limitations of renal function tests. Semin Nucl Med. 2008;38:32–46. - PubMed
-
- Blaufox MD, Aurell M, Bubeck B, Fommei E, Piepsz A, Russell C, et al. Report of the radionuclides in nephrourology committee on renal clearance. J Nucl Med. 1996;37:1883–90. - PubMed
-
- Shikano N, Kanai Y, Kawai K, Ishikawa N, Endou H. Transport of 99mTc-MAG3 via rat renal organic anion transporter 1. J Nucl Med. 2004;45:80–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

