The complexity of high-frequency electric fields degrades electrosensory inputs: implications for the jamming avoidance response in weakly electric fish
- PMID: 29367237
- PMCID: PMC5805966
- DOI: 10.1098/rsif.2017.0633
The complexity of high-frequency electric fields degrades electrosensory inputs: implications for the jamming avoidance response in weakly electric fish
Abstract
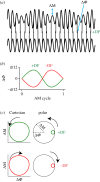
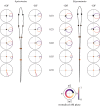
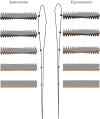
Sensory systems encode environmental information that is necessary for adaptive behavioural choices, and thus greatly influence the evolution of animal behaviour and the underlying neural circuits. Here, we evaluate how the quality of sensory information impacts the jamming avoidance response (JAR) in weakly electric fish. To sense their environment, these fish generate an oscillating electric field: the electric organ discharge (EOD). Nearby fish with similar EOD frequencies perform the JAR to increase the difference between their EOD frequencies, i.e. their difference frequency (DF). The fish determines the sign of the DF: when it has a lower frequency (DF > 0), EOD frequency is decreased and vice versa. We study the sensory basis of the JAR in two species: Apteronotus leptorhynchus have a high frequency (ca 1000 Hz), spatio-temporally heterogeneous electric field, whereas Eigenmannia sp. have a low frequency (ca 300 Hz), spatially uniform field. We show that the increased complexity of the Apteronotus field decreases the reliability of sensory cues used to determine the DF. Interestingly, Apteronotus responds to all JAR stimuli by increasing EOD frequency, having lost the neural pathway that produces JAR-related decreases in EOD frequency. Our results suggest that electric field complexity may have influenced the evolution of the JAR by degrading the related sensory information.
Keywords: Apteronotus; Eigenmannia; amplitude modulation; electrocommunication; signal interference.
© 2018 The Author(s).
Conflict of interest statement
We declare we have no competing interests
Figures





References
-
- Moller P. 1995. Electric fishes: history and behavior. London, UK: Chapman & Hall.
-
- Lissmann HW. 1958. On the function and evolution of electric organs in fish. J. Exp. Biol. 35, 156–191.
-
- Bennett M. 1971. Electroreception. In Fish physiology (eds WS Hoar, DL Randall), pp. 493–574. New: York, NY: Academic Press.
-
- Heiligenberg W, Baker C, Matsubara J. 1978. The jamming avoidance response in Eigenmannia revisited: the structure of a neuronal democracy. J. Comp. Physiol. 127, 267–286. ( 10.1007/BF01350117) - DOI
-
- Heiligenberg W. 1991. Neural nets in electric fish. Cambridge, MA: MIT Press.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
