Towards personalized computational oncology: from spatial models of tumour spheroids, to organoids, to tissues
- PMID: 29367239
- PMCID: PMC5805971
- DOI: 10.1098/rsif.2017.0703
Towards personalized computational oncology: from spatial models of tumour spheroids, to organoids, to tissues
Abstract
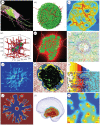
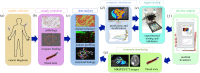
A main goal of mathematical and computational oncology is to develop quantitative tools to determine the most effective therapies for each individual patient. This involves predicting the right drug to be administered at the right time and at the right dose. Such an approach is known as precision medicine. Mathematical modelling can play an invaluable role in the development of such therapeutic strategies, since it allows for relatively fast, efficient and inexpensive simulations of a large number of treatment schedules in order to find the most effective. This review is a survey of mathematical models that explicitly take into account the spatial architecture of three-dimensional tumours and address tumour development, progression and response to treatments. In particular, we discuss models of epithelial acini, multicellular spheroids, normal and tumour spheroids and organoids, and multi-component tissues. Our intent is to showcase how these in silico models can be applied to patient-specific data to assess which therapeutic strategies will be the most efficient. We also present the concept of virtual clinical trials that integrate standard-of-care patient data, medical imaging, organ-on-chip experiments and computational models to determine personalized medical treatment strategies.
Keywords: agent-based models; cancer treatment; mathematical modelling; mathematical oncology; virtual clinical trials.
© 2018 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Desmond-Hellmann S, et al. 2011. Toward precision medicine: building a knowledge network for biomedical research and a new taxonomy of disease. Washington, DC: The National Academies Press. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
