Purification of Highly Active Alphavirus Replication Complexes Demonstrates Altered Fractionation of Multiple Cellular Membranes
- PMID: 29367248
- PMCID: PMC5874421
- DOI: 10.1128/JVI.01852-17
Purification of Highly Active Alphavirus Replication Complexes Demonstrates Altered Fractionation of Multiple Cellular Membranes
Abstract
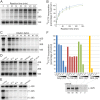
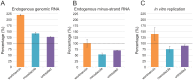
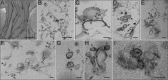
Positive-strand RNA viruses replicate their genomes in membrane-associated structures; alphaviruses and many other groups induce membrane invaginations called spherules. Here, we established a protocol to purify these membranous replication complexes (RCs) from cells infected with Semliki Forest virus (SFV). We isolated SFV spherules located on the plasma membrane and further purified them using two consecutive density gradients. This revealed that SFV infection strongly modifies cellular membranes. We removed soluble proteins, the Golgi membranes, and most of the mitochondria, but plasma membrane, endoplasmic reticulum (ER), and late endosome markers were retained in the membrane fraction that contained viral RNA synthesizing activity, replicase proteins, and minus- and plus-strand RNA. Electron microscopy revealed that the purified membranes displayed spherule-like structures with a narrow neck. This membrane enrichment was specific to viral replication, as such a distribution of membrane markers was only observed after infection. Besides the plasma membrane, SFV infection remodeled the ER, and the cofractionation of the RC-carrying plasma membrane and ER suggests that SFV recruits ER proteins or membrane to the site of replication. The purified RCs were highly active in synthesizing both genomic and subgenomic RNA. Detergent solubilization destroyed the replication activity, demonstrating that the membrane association of the complex is essential. Most of the newly made RNA was in double-stranded replicative molecules, but the purified complexes also produced single-stranded RNA as well as released newly made RNA. This indicates that the purification established here maintained the functionality of RCs and thus enables further structural and functional studies of active RCs.IMPORTANCE Similar to all positive-strand RNA viruses, the arthropod-borne alphaviruses induce membranous genome factories, but little is known about the arrangement of viral replicase proteins and the presence of host proteins in these replication complexes. To improve our knowledge of alphavirus RNA-synthesizing complexes, we isolated and purified them from infected mammalian cells. Detection of viral RNA and in vitro replication assays revealed that these complexes are abundant and highly active when located on the plasma membrane. After multiple purification steps, they remain functional in synthesizing and releasing viral RNA. Besides the plasma membrane, markers for the endoplasmic reticulum and late endosomes were enriched with the replication complexes, demonstrating that alphavirus infection modified cellular membranes beyond inducing replication spherules on the plasma membrane. We have developed here a gentle purification method to obtain large quantities of highly active replication complexes, and similar methods can be applied to other positive-strand RNA viruses.
Keywords: RNA replication; Semliki Forest virus; alphavirus; membrane fractionation; purification; replication complex.
Copyright © 2018 American Society for Microbiology.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

