MALT1 Controls Attenuated Rabies Virus by Inducing Early Inflammation and T Cell Activation in the Brain
- PMID: 29367251
- PMCID: PMC5874405
- DOI: 10.1128/JVI.02029-17
MALT1 Controls Attenuated Rabies Virus by Inducing Early Inflammation and T Cell Activation in the Brain
Abstract
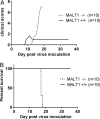
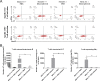
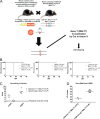
MALT1 is involved in the activation of immune responses, as well as in the proliferation and survival of certain cancer cells. MALT1 acts as a scaffold protein for NF-κB signaling and a cysteine protease that cleaves substrates, further promoting the expression of immunoregulatory genes. Deregulated MALT1 activity has been associated with autoimmunity and cancer, implicating MALT1 as a new therapeutic target. Although MALT1 deficiency has been shown to protect against experimental autoimmune encephalomyelitis, nothing is known about the impact of MALT1 on virus infection in the central nervous system. Here, we studied infection with an attenuated rabies virus, Evelyn-Rotnycki-Abelseth (ERA) virus, and observed increased susceptibility with ERA virus in MALT1-/- mice. Indeed, after intranasal infection with ERA virus, wild-type mice developed mild transient clinical signs with recovery at 35 days postinoculation (dpi). Interestingly, MALT1-/- mice developed severe disease requiring euthanasia at around 17 dpi. A decreased induction of inflammatory gene expression and cell infiltration and activation was observed in MALT1-/- mice at 10 dpi compared to MALT1+/+ infected mice. At 17 dpi, however, the level of inflammatory cell activation was comparable to that observed in MALT1+/+ mice. Moreover, MALT1-/- mice failed to produce virus-neutralizing antibodies. Similar results were obtained with specific inactivation of MALT1 in T cells. Finally, treatment of wild-type mice with mepazine, a MALT1 protease inhibitor, also led to mortality upon ERA virus infection. These data emphasize the importance of early inflammation and activation of T cells through MALT1 for controlling the virulence of an attenuated rabies virus in the brain.IMPORTANCE Rabies virus is a neurotropic virus which can infect any mammal. Annually, 59,000 people die from rabies. Effective therapy is lacking and hampered by gaps in the understanding of virus pathogenicity. MALT1 is an intracellular protein involved in innate and adaptive immunity and is an interesting therapeutic target because MALT1-deregulated activity has been associated with autoimmunity and cancers. The role of MALT1 in viral infection is, however, largely unknown. Here, we study the impact of MALT1 on virus infection in the brain, using the attenuated ERA rabies virus in different models of MALT1-deficient mice. We reveal the importance of MALT1-mediated inflammation and T cell activation to control ERA virus, providing new insights in the biology of MALT1 and rabies virus infection.
Keywords: ERA; MALT1; immunity; neuroinflammation; rabies virus.
Copyright © 2018 Kip et al.
Figures








Similar articles
-
MALT1 Proteolytic Activity Suppresses Autoimmunity in a T Cell Intrinsic Manner.Front Immunol. 2019 Aug 14;10:1898. doi: 10.3389/fimmu.2019.01898. eCollection 2019. Front Immunol. 2019. PMID: 31474984 Free PMC article.
-
Inhibition of MALT1 Decreases Neuroinflammation and Pathogenicity of Virulent Rabies Virus in Mice.J Virol. 2018 Oct 29;92(22):e00720-18. doi: 10.1128/JVI.00720-18. Print 2018 Nov 15. J Virol. 2018. PMID: 30158289 Free PMC article.
-
Inflammatory responses in the nervous system of mice infected with a street isolate of rabies virus.Dev Biol (Basel). 2008;131:65-72. Dev Biol (Basel). 2008. PMID: 18634467
-
MALT1--a universal soldier: multiple strategies to ensure NF-κB activation and target gene expression.FEBS J. 2015 Sep;282(17):3286-97. doi: 10.1111/febs.13325. Epub 2015 Jun 10. FEBS J. 2015. PMID: 25996250 Review.
-
Targeting MALT1 Proteolytic Activity in Immunity, Inflammation and Disease: Good or Bad?Trends Mol Med. 2016 Feb;22(2):135-150. doi: 10.1016/j.molmed.2015.12.004. Epub 2016 Jan 17. Trends Mol Med. 2016. PMID: 26787500 Review.
Cited by
-
Identification of an Ortholog of MALT1 from Shrimp That Induces NF-κB-Mediated Antiviral Immunity.Viruses. 2023 Nov 30;15(12):2361. doi: 10.3390/v15122361. Viruses. 2023. PMID: 38140602 Free PMC article.
-
PRRSV utilizes MALT1-regulated autophagy flux to switch virus spread and reserve.Autophagy. 2024 Dec;20(12):2697-2718. doi: 10.1080/15548627.2024.2386195. Epub 2024 Aug 6. Autophagy. 2024. PMID: 39081059 Free PMC article.
-
MALT1 Proteolytic Activity Suppresses Autoimmunity in a T Cell Intrinsic Manner.Front Immunol. 2019 Aug 14;10:1898. doi: 10.3389/fimmu.2019.01898. eCollection 2019. Front Immunol. 2019. PMID: 31474984 Free PMC article.
-
MALT1 in asthma children: A potential biomarker for monitoring exacerbation risk and Th1/Th2 imbalance-mediated inflammation.J Clin Lab Anal. 2022 May;36(5):e24379. doi: 10.1002/jcla.24379. Epub 2022 Mar 30. J Clin Lab Anal. 2022. PMID: 35353938 Free PMC article.
-
Dual Role of Toll-Like Receptor 7 in the Pathogenesis of Rabies Virus in a Mouse Model.J Virol. 2020 Apr 16;94(9):e00111-20. doi: 10.1128/JVI.00111-20. Print 2020 Apr 16. J Virol. 2020. PMID: 32102880 Free PMC article.
References
-
- Hailfinger S, Nogai H, Pelzer C, Jaworski M, Cabalzar K, Charton JE, Guzzardi M, Decaillet C, Grau M, Dorken B, Lenz P, Lenz G, Thome M. 2011. Malt1-dependent RelB cleavage promotes canonical NF-κB activation in lymphocytes and lymphoma cell lines. Proc Natl Acad Sci U S A 108:14596–14601. doi:10.1073/pnas.1105020108. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

