Biogenesis of zinc storage granules in Drosophila melanogaster
- PMID: 29367274
- PMCID: PMC5897703
- DOI: 10.1242/jeb.168419
Biogenesis of zinc storage granules in Drosophila melanogaster
Abstract
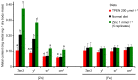
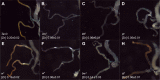
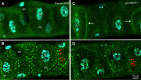
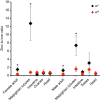
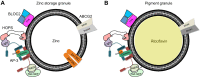
Membrane transporters and sequestration mechanisms concentrate metal ions differentially into discrete subcellular microenvironments for use in protein cofactors, signalling, storage or excretion. Here we identify zinc storage granules as the insect's major zinc reservoir in principal Malpighian tubule epithelial cells of Drosophila melanogaster The concerted action of Adaptor Protein-3, Rab32, HOPS and BLOC complexes as well as of the white-scarlet (ABCG2-like) and ZnT35C (ZnT2/ZnT3/ZnT8-like) transporters is required for zinc storage granule biogenesis. Due to lysosome-related organelle defects caused by mutations in the homologous human genes, patients with Hermansky-Pudlak syndrome may lack zinc granules in beta pancreatic cells, intestinal paneth cells and presynaptic vesicles of hippocampal mossy fibers.
Keywords: AP-3 complex; Eye color mutants; ICP-OES; Malpighian tubules; Synchrotron; Zincosomes.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
-
- Ammann S., Schulz A., Krageloh-Mann I., Dieckmann N. M., Niethammer K., Fuchs S., Eckl K. M., Plank R., Werner R., Altmuller J. et al. (2016). Mutations in AP3D1 associated with immunodeficiency and seizures define a new type of Hermansky-Pudlak syndrome. Blood 127, 997-1006. 10.1182/blood-2015-09-671636 - DOI - PMC - PubMed
-
- Assoum M., Philippe C., Isidor B., Perrin L., Makrythanasis P., Sondheimer N., Paris C., Douglas J., Lesca G., Antonarakis S. et al. (2016). Autosomal-recessive mutations in AP3B2, adaptor-related protein complex 3 β2 subunit, cause an early-onset epileptic encephalopathy with optic atrophy. Am. J. Hum. Genet. 99, 1368-1376. 10.1016/j.ajhg.2016.10.009 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

