How Forces Fold the Cerebral Cortex
- PMID: 29367287
- PMCID: PMC5783962
- DOI: 10.1523/JNEUROSCI.1105-17.2017
How Forces Fold the Cerebral Cortex
Abstract
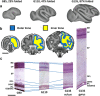
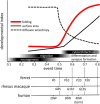
Improved understanding of the factors that govern folding of the cerebral cortex is desirable for many reasons. The existence of consistent patterns in folding within and between species suggests a fundamental role in brain function. Abnormal folding patterns found in individuals affected by a diverse array of neurodevelopmental disorders underline the clinical relevance of understanding the folding process. Recent experimental and computational efforts to elucidate the biomechanical forces involved in cerebral cortical folding have converged on a consistent approach. Brain growth is modeled with two components: an expanding outer zone, destined to become the cerebral cortex, is mechanically coupled to an inner zone, destined to become white matter, that grows at a slower rate, perhaps in response to stress induced by expansion from the outer layer. This framework is consistent with experimentally observed internal forces in developing brains, and with observations of the folding process in physical models. In addition, computational simulations based on this foundation can produce folding patterns that recapitulate the characteristics of folding patterns found in gyroencephalic brains. This perspective establishes the importance of mechanical forces in our current understanding of how brains fold, and identifies realistic ranges for specific parameters in biophysical models of developing brain tissue. However, further refinement of this approach is needed. An understanding of mechanical forces that arise during brain development and their cellular-level origins is necessary to interpret the consequences of abnormal brain folding and its role in functional deficits as well as neurodevelopmental disease.Dual Perspectives Companion Paper: How Cells Fold the Cerebral Cortex, by Víctor Borrell.
Keywords: biomechanics; development; fetal brain; gyrus; morphology.
Copyright © 2018 the authors 0270-6474/18/380767-09$15.00/0.
Figures
References
-
- Barkovich AJ, Raybaud C (2012) Pediatric neuroimaging. Philadelphia, PA: Lippincott Williams and Wilkins.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
