Defining the binding determinants of Shewanella oneidensis OxyR: Implications for the link between the contracted OxyR regulon and adaptation
- PMID: 29367341
- PMCID: PMC5858001
- DOI: 10.1074/jbc.RA117.001530
Defining the binding determinants of Shewanella oneidensis OxyR: Implications for the link between the contracted OxyR regulon and adaptation
Abstract
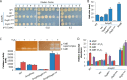
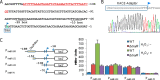
It is well-established that OxyR functions as a transcriptional activator of the peroxide stress response in bacteria, primarily based on studies on Escherichia coli Recent investigations have revealed that OxyRs of some other bacteria can regulate gene expression through both repression and activation or repression only; however, the underlying mechanisms remain largely unknown. Here, we demonstrated in γ-proteobacteriumShewanella oneidensis regulation of OxyR on expression of major catalase gene katB in a dual-control manner through interaction with a single site in the promoter region. Under non-stress conditions, katB expression was repressed by reduced OxyR (OxyRred), whereas when oxidized, OxyR (OxyRoxi) outcompeted OxyRred for the site because of substantially enhanced affinity, resulting in a graded response to oxidative stress, from repression to derepression to activation. The OxyR-binding motif is characterized as a combination of the E. coli motif (tetranucleotides spaced by heptanucleotide) and palindromic structure. We provided evidence to suggest that the S. oneidensis OxyR regulon is significantly contracted compared with those reported, probably containing only five members that are exclusively involved in oxygen reactive species scavenging and iron sequestering. These characteristics probably reflect the adapting strategy of the bacteria that S. oneidensis represents to thrive in redox-stratified microaerobic and anaerobic environments.
Keywords: DNA binding protein; bacterial genetics; gene regulation; hydrogen peroxide; oxidative stress.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

