GPR56/ADGRG1 regulates development and maintenance of peripheral myelin
- PMID: 29367382
- PMCID: PMC5839751
- DOI: 10.1084/jem.20161714
GPR56/ADGRG1 regulates development and maintenance of peripheral myelin
Abstract
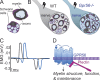
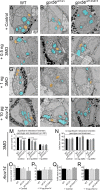
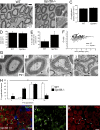
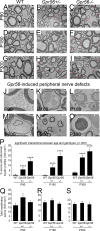
Myelin is a multilamellar sheath generated by specialized glia called Schwann cells (SCs) in the peripheral nervous system (PNS), which serves to protect and insulate axons for rapid neuronal signaling. In zebrafish and rodent models, we identify GPR56/ADGRG1 as a conserved regulator of PNS development and health. We demonstrate that, during SC development, GPR56-dependent RhoA signaling promotes timely radial sorting of axons. In the mature PNS, GPR56 is localized to distinct SC cytoplasmic domains, is required to establish proper myelin thickness, and facilitates organization of the myelin sheath. Furthermore, we define plectin-a scaffolding protein previously linked to SC domain organization, myelin maintenance, and a series of disorders termed "plectinopathies"-as a novel interacting partner of GPR56. Finally, we show that Gpr56 mutants develop progressive neuropathy-like symptoms, suggesting an underlying mechanism for peripheral defects in some human patients with GPR56 mutations. In sum, we define Gpr56 as a new regulator in the development and maintenance of peripheral myelin.
© 2018 Ackerman et al.
Figures




References
-
- An, B., Zhang M., Johnson R.W., and Qu J.. 2015. Surfactant-aided precipitation/on-pellet-digestion (SOD) procedure provides robust and rapid sample preparation for reproducible, accurate and sensitive LC/MS quantification of therapeutic protein in plasma and tissues. Anal. Chem. 87:4023–4029. 10.1021/acs.analchem.5b00350 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

