Cortical Neural Activity Predicts Sensory Acuity Under Optogenetic Manipulation
- PMID: 29367406
- PMCID: PMC5824743
- DOI: 10.1523/JNEUROSCI.2457-17.2017
Cortical Neural Activity Predicts Sensory Acuity Under Optogenetic Manipulation
Abstract
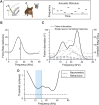
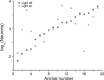
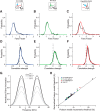
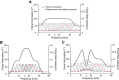
Excitatory and inhibitory neurons in the mammalian sensory cortex form interconnected circuits that control cortical stimulus selectivity and sensory acuity. Theoretical studies have predicted that suppression of inhibition in such excitatory-inhibitory networks can lead to either an increase or, paradoxically, a decrease in excitatory neuronal firing, with consequent effects on stimulus selectivity. We tested whether modulation of inhibition or excitation in the auditory cortex of male mice could evoke such a variety of effects in tone-evoked responses and in behavioral frequency discrimination acuity. We found that, indeed, the effects of optogenetic manipulation on stimulus selectivity and behavior varied in both magnitude and sign across subjects, possibly reflecting differences in circuitry or expression of optogenetic factors. Changes in neural population responses consistently predicted behavioral changes for individuals separately, including improvement and impairment in acuity. This correlation between cortical and behavioral change demonstrates that, despite the complex and varied effects that these manipulations can have on neuronal dynamics, the resulting changes in cortical activity account for accompanying changes in behavioral acuity.SIGNIFICANCE STATEMENT Excitatory and inhibitory interactions determine stimulus specificity and tuning in sensory cortex, thereby controlling perceptual discrimination acuity. Modeling has predicted that suppressing the activity of inhibitory neurons can lead to increased or, paradoxically, decreased excitatory activity depending on the architecture of the network. Here, we capitalized on differences between subjects to test whether suppressing/activating inhibition and excitation can in fact exhibit such paradoxical effects for both stimulus sensitivity and behavioral discriminability. Indeed, the same optogenetic manipulation in the auditory cortex of different mice could improve or impair frequency discrimination acuity, predictable from the effects on cortical responses to tones. The same manipulations sometimes produced opposite changes in the behavior of different individuals, supporting theoretical predictions for inhibition-stabilized networks.
Keywords: auditory cortex; behavior; computational modeling; excitatory–inhibitory circuits; frequency discrimination; optogenetics.
Copyright © 2018 the authors 0270-6474/18/382094-12$15.00/0.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
