Recombinant Human ADAMTS13 Treatment Improves Myocardial Remodeling and Functionality After Pressure Overload Injury in Mice
- PMID: 29367415
- PMCID: PMC5850234
- DOI: 10.1161/JAHA.117.007004
Recombinant Human ADAMTS13 Treatment Improves Myocardial Remodeling and Functionality After Pressure Overload Injury in Mice
Abstract
Background: A disintegrin-like metalloproteinase with thrombospondin motif type 1 member 13 (ADAMTS13), the von Willebrand factor-cleaving enzyme, decreases leukocyte and platelet recruitment and, thus, reduces thrombosis and inflammation. Recombinant human ADAMTS13 (rhADAMTS13) is a novel drug candidate for ischemia/reperfusion injury and has shown short-term benefits in mouse models of myocardial injury, but long-term outcome has not been investigated.
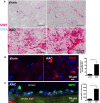
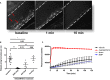
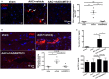
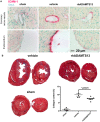
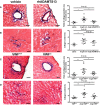
Methods and results: We evaluated the impact of rhADAMTS13 on cardiac remodeling, scarring, and contractile function, under chronic left ventricular pressure overload. The role of von Willebrand factor and the effect of rhADAMTS13 treatment were studied. This model of heart failure, based on ascending aortic constriction, produces a coronary inflammatory response and microvascular dysfunction, resulting in fibrotic remodeling and cardiac failure. Mice were treated with either rhADAMTS13 or vehicle and assessed for coronary vascular inflammation and ventricular function at several postsurgical time points, as well as for cardiac fibrosis after 4 weeks. Early upon induction of pressure overload under rhADAMTS13 treatment, we detected less endothelial-lumen-associated von Willebrand factor, fewer platelet aggregates, and decreased activated transforming growth factor-β1 levels than in vehicle-treated mice. We observed significant preservation of cardiac function and decrease in fibrotic remodeling as a result of rhADAMTS13 administration.
Conclusions: Herein, we show that rhADAMTS13 decreases coronary vascular dysfunction and improves cardiac remodeling after left ventricular pressure overload in mice. We propose that this effect may, at least in part, be the result of decreased von Willebrand factor-mediated recruitment of platelets, a major source of the activated profibrotic cytokine transforming growth factor-β1. Our study further supports the therapeutic potential of rhADAMTS13 for conditions characterized by inflammatory cardiac damage that results in fibrosis.
Keywords: ADAMTS13; cardiac remodeling; fibrosis; heart failure; von Willebrand factor.
© 2018 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures

References
-
- Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

