Chemotherapy induces enrichment of CD47+/CD73+/PDL1+ immune evasive triple-negative breast cancer cells
- PMID: 29367423
- PMCID: PMC5819443
- DOI: 10.1073/pnas.1718197115
Chemotherapy induces enrichment of CD47+/CD73+/PDL1+ immune evasive triple-negative breast cancer cells
Abstract
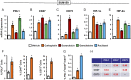
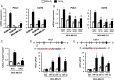
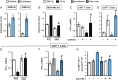
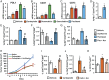
Triple-negative breast cancer (TNBC) is treated with cytotoxic chemotherapy and is often characterized by early relapse and metastasis. To form a secondary (recurrent and/or metastatic) tumor, a breast cancer cell must evade the innate and adaptive immune systems. CD47 enables cancer cells to evade killing by macrophages, whereas CD73 and PDL1 mediate independent mechanisms of evasion of cytotoxic T lymphocytes. Here, we report that treatment of human or murine TNBC cells with carboplatin, doxorubicin, gemcitabine, or paclitaxel induces the coordinate transcriptional induction of CD47, CD73, and PDL1 mRNA and protein expression, leading to a marked increase in the percentage of CD47+CD73+PDL1+ breast cancer cells. Genetic or pharmacological inhibition of hypoxia-inducible factors (HIFs) blocked chemotherapy-induced enrichment of CD47+CD73+PDL1+ TNBC cells, which were also enriched in the absence of chemotherapy by incubation under hypoxic conditions, leading to T cell anergy or death. Treatment of mice with cytotoxic chemotherapy markedly increased the intratumoral ratio of regulatory/effector T cells, an effect that was abrogated by HIF inhibition. Our results delineate an HIF-dependent transcriptional mechanism contributing to TNBC progression and suggest that combining chemotherapy with an HIF inhibitor may prevent countertherapeutic induction of proteins that mediate evasion of innate and adaptive antitumor immunity.
Keywords: HIF-1; PD-1; adenosine; effector T cells; regulatory T cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Newman LA, Reis-Filho JS, Morrow M, Carey LA, King TA. The 2014 Society of Surgical Oncology Susan G. Komen for the Cure Symposium: Triple-negative breast cancer. Ann Surg Oncol. 2015;22:874–882. - PubMed
-
- Gadi VK, Davidson NE. Practical approach to triple-negative breast cancer. J Oncol Pract. 2017;13:293–300. - PubMed
-
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

