Interaction between the Caenorhabditis elegans centriolar protein SAS-5 and microtubules facilitates organelle assembly
- PMID: 29367435
- PMCID: PMC6003225
- DOI: 10.1091/mbc.E17-06-0412
Interaction between the Caenorhabditis elegans centriolar protein SAS-5 and microtubules facilitates organelle assembly
Abstract
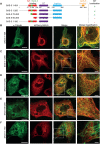
Centrioles are microtubule-based organelles that organize the microtubule network and seed the formation of cilia and flagella. New centrioles assemble through a stepwise process dependent notably on the centriolar protein SAS-5 in Caenorhabditis elegans SAS-5 and its functional homologues in other species form oligomers that bind the centriolar proteins SAS-6 and SAS-4, thereby forming an evolutionarily conserved structural core at the onset of organelle assembly. Here, we report a novel interaction of SAS-5 with microtubules. Microtubule binding requires SAS-5 oligomerization and a disordered protein segment that overlaps with the SAS-4 binding site. Combined in vitro and in vivo analysis of select mutants reveals that the SAS-5-microtubule interaction facilitates centriole assembly in C. elegans embryos. Our findings lead us to propose that the interdependence of SAS-5 oligomerization and microtubule binding reflects an avidity mechanism, which also strengthens SAS-5 associations with other centriole components and, thus, promotes organelle assembly.
© 2018 Bianchi, Rogala, Dynes, et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

