Noninvasive gene delivery to foveal cones for vision restoration
- PMID: 29367457
- PMCID: PMC5821199
- DOI: 10.1172/jci.insight.96029
Noninvasive gene delivery to foveal cones for vision restoration
Abstract
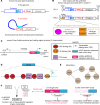
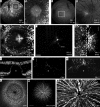
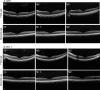
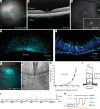
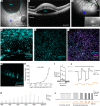
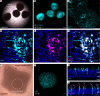
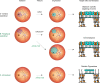
Intraocular injection of adeno-associated viral (AAV) vectors has been an evident route for delivering gene drugs into the retina. However, gaps in our understanding of AAV transduction patterns within the anatomically unique environments of the subretinal and intravitreal space of the primate eye impeded the establishment of noninvasive and efficient gene delivery to foveal cones in the clinic. Here, we establish new vector-promoter combinations to overcome the limitations associated with AAV-mediated cone transduction in the fovea with supporting studies in mouse models, human induced pluripotent stem cell-derived organoids, postmortem human retinal explants, and living macaques. We show that an AAV9 variant provides efficient foveal cone transduction when injected into the subretinal space several millimeters away from the fovea, without detaching this delicate region. An engineered AAV2 variant provides gene delivery to foveal cones with a well-tolerated dose administered intravitreally. Both delivery modalities rely on a cone-specific promoter and result in high-level transgene expression compatible with optogenetic vision restoration. The model systems described here provide insight into the behavior of AAV vectors across species to obtain safety and efficacy needed for gene therapy in neurodegenerative disorders.
Keywords: Gene therapy; Genetic diseases; Ophthalmology; Surgery; Therapeutics.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

