Mutations in Hnrnpa1 cause congenital heart defects
- PMID: 29367466
- PMCID: PMC5821217
- DOI: 10.1172/jci.insight.98555
Mutations in Hnrnpa1 cause congenital heart defects
Abstract
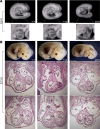
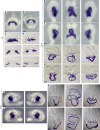
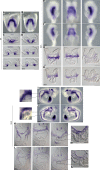
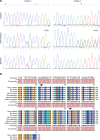
Incomplete penetrance of congenital heart defects (CHDs) was observed in a mouse model. We hypothesized that the contribution of a major genetic locus modulates the manifestation of the CHDs. After genome-wide linkage mapping, fine mapping, and high-throughput targeted sequencing, a recessive frameshift mutation of the heterogeneous nuclear ribonucleoprotein A1 (Hnrnpa1) gene was confirmed (Hnrnpa1ct). Hnrnpa1 was expressed in both the first heart field (FHF) and second heart field (SHF) at the cardiac crescent stage but was only maintained in SHF progenitors after heart tube formation. Hnrnpa1ct/ct homozygous mutants displayed complete CHD penetrance, including truncated and incomplete looped heart tube at E9.5, ventricular septal defect (VSD) and persistent truncus arteriosus (PTA) at E13.5, and VSD and double outlet right ventricle at P0. Impaired development of the dorsal mesocardium and sinoatrial node progenitors was also observed. Loss of Hnrnpa1 expression leads to dysregulation of cardiac transcription networks and multiple signaling pathways, including BMP, FGF, and Notch in the SHF. Finally, two rare heterozygous mutations of HNRNPA1 were detected in human CHDs. These findings suggest a role of Hnrnpa1 in embryonic heart development in mice and humans.
Keywords: Cardiovascular disease; Development; Genetics; Heart failure; Organogenesis.
Conflict of interest statement
Figures




Similar articles
-
HAND transcription factors cooperatively specify the aorta and pulmonary trunk.Dev Biol. 2021 Aug;476:1-10. doi: 10.1016/j.ydbio.2021.03.011. Epub 2021 Mar 20. Dev Biol. 2021. PMID: 33757801 Free PMC article.
-
Rac1 signaling is critical to cardiomyocyte polarity and embryonic heart development.J Am Heart Assoc. 2014 Oct 14;3(5):e001271. doi: 10.1161/JAHA.114.001271. J Am Heart Assoc. 2014. PMID: 25315346 Free PMC article.
-
SHROOM3 is downstream of the planar cell polarity pathway and loss-of-function results in congenital heart defects.Dev Biol. 2020 Aug 15;464(2):124-136. doi: 10.1016/j.ydbio.2020.05.013. Epub 2020 Jun 5. Dev Biol. 2020. PMID: 32511952 Free PMC article.
-
Genetic regulation of cardiogenesis and congenital heart disease.Annu Rev Pathol. 2006;1:199-213. doi: 10.1146/annurev.pathol.1.110304.100039. Annu Rev Pathol. 2006. PMID: 18039113 Review.
-
An overview of cardiac morphogenesis.Arch Cardiovasc Dis. 2013 Nov;106(11):612-23. doi: 10.1016/j.acvd.2013.07.001. Epub 2013 Oct 15. Arch Cardiovasc Dis. 2013. PMID: 24138816 Review.
Cited by
-
RNA Modification by m6A Methylation in Cardiovascular Disease.Oxid Med Cell Longev. 2021 Feb 9;2021:8813909. doi: 10.1155/2021/8813909. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34221238 Free PMC article. Review.
-
A novel TBX5 mutation predisposes to familial cardiac septal defects and atrial fibrillation as well as bicuspid aortic valve.Genet Mol Biol. 2020 Nov 13;43(4):e20200142. doi: 10.1590/1678-4685-GMB-2020-0142. eCollection 2020. Genet Mol Biol. 2020. PMID: 33306779 Free PMC article.
-
RNA binding proteins in cardiovascular development and disease.Curr Top Dev Biol. 2024;156:51-119. doi: 10.1016/bs.ctdb.2024.01.007. Epub 2024 Mar 15. Curr Top Dev Biol. 2024. PMID: 38556427 Free PMC article.
-
Genetic Animal Models of Cardiovascular Pathologies.Biomedicines. 2025 Jun 21;13(7):1518. doi: 10.3390/biomedicines13071518. Biomedicines. 2025. PMID: 40722594 Free PMC article. Review.
-
Alternative splicing factors and cardiac disease: more than just missplicing?RNA. 2025 Feb 19;31(3):300-306. doi: 10.1261/rna.080332.124. RNA. 2025. PMID: 39773891 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

