Lymphatic function measurements influenced by contrast agent volume and body position
- PMID: 29367467
- PMCID: PMC5821192
- DOI: 10.1172/jci.insight.96591
Lymphatic function measurements influenced by contrast agent volume and body position
Abstract
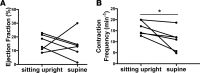
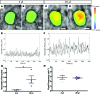
Several imaging modalities have been used to assess lymphatic function, including fluorescence microscopy, near-infrared fluorescence (NIRF) imaging, and Doppler optical coherence tomography (DOCT). They vary in how the mouse is positioned, the invasiveness of the experimental setup, and the volume of contrast agent injected. Here, we present how each of these experimental parameters affects functional measurements of collecting lymphatic vessels. First, fluorescence microscopy showed that supine mice have a statistically lower contraction frequency compared with mice sitting upright. To assess the effect of different injection volumes on these endpoints, mice were injected with 4, 10, or 20 μl of dye. The lowest frequencies were observed after 20-μl injections. Interestingly, lymph-flow DOCT revealed that although there was lower contraction frequency in mice injected with 20 μl versus 4 μl, mice showed a higher volumetric flow with a 20-μl injection. This indicates that contraction frequency alone is not sufficient to understand lymphatic transport. Finally, NIRF revealed that removing the skin reduced contraction frequency. Therefore, this study reveals how sensitive these techniques are to mouse position, removal of skin, and dye volume. Care should be taken when comparing results obtained under different experimental conditions.
Keywords: Lymph; Vascular Biology.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

