Lineages of the Cardiac Conduction System
- PMID: 29367537
- PMCID: PMC5715704
- DOI: 10.3390/jcdd4020005
Lineages of the Cardiac Conduction System
Abstract
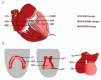
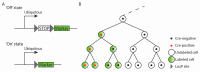
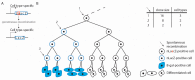
The cardiac conduction system (CCS) initiates and coordinately propagates the electrical impulse to orchestrate the heartbeat. It consists of a set of interconnected components with shared properties. A better understanding of the origin and specification of CCS lineages has allowed us to better comprehend the etiology of CCS disease and has provided leads for development of therapies. A variety of technologies and approaches have been used to investigate CCS lineages, which will be summarized in this review. The findings imply that there is not a single CCS lineage. In contrast, early cell fate decisions segregate the lineages of the CCS components while they remain connected to each other.
Keywords: cardiac conduction system; cardiac conduction system lineage; cardiac development; cell lineage; genetic fate map; genetic inducible fate map; lineage tracing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

