Electric fields control the orientation of peptides irreversibly immobilized on radical-functionalized surfaces
- PMID: 29367659
- PMCID: PMC5783936
- DOI: 10.1038/s41467-017-02545-6
Electric fields control the orientation of peptides irreversibly immobilized on radical-functionalized surfaces
Abstract
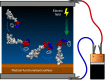
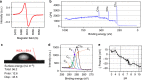
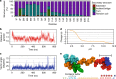
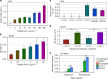
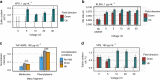
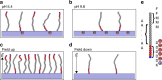
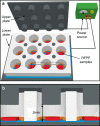
Surface functionalization of an implantable device with bioactive molecules can overcome adverse biological responses by promoting specific local tissue integration. Bioactive peptides have advantages over larger protein molecules due to their robustness and sterilizability. Their relatively small size presents opportunities to control the peptide orientation on approach to a surface to achieve favourable presentation of bioactive motifs. Here we demonstrate control of the orientation of surface-bound peptides by tuning electric fields at the surface during immobilization. Guided by computational simulations, a peptide with a linear conformation in solution is designed. Electric fields are used to control the peptide approach towards a radical-functionalized surface. Spontaneous, irreversible immobilization is achieved when the peptide makes contact with the surface. Our findings show that control of both peptide orientation and surface concentration is achieved simply by varying the solution pH or by applying an electric field as delivered by a small battery.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Dias, J., Kay, P., Porter, M. & Briggs, T. Restoring Your Mobility. Available at http://www.boa.ac.uk/wp-content/uploads/2014/01/BOA-Practise-Strategy.pdf (British Orthopaedic Association, 2012).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

