A comparison of sample preparation methods for extracting volatile organic compounds (VOCs) from equine faeces using HS-SPME
- PMID: 29367839
- PMCID: PMC5754382
- DOI: 10.1007/s11306-017-1315-7
A comparison of sample preparation methods for extracting volatile organic compounds (VOCs) from equine faeces using HS-SPME
Abstract
Introduction: Disturbance to the hindgut microbiota can be detrimental to equine health. Metabolomics provides a robust approach to studying the functional aspect of hindgut microorganisms. Sample preparation is an important step towards achieving optimal results in the later stages of analysis. The preparation of samples is unique depending on the technique employed and the sample matrix to be analysed. Gas chromatography mass spectrometry (GCMS) is one of the most widely used platforms for the study of metabolomics and until now an optimised method has not been developed for equine faeces.
Objectives: To compare a sample preparation method for extracting volatile organic compounds (VOCs) from equine faeces.
Methods: Volatile organic compounds were determined by headspace solid phase microextraction gas chromatography mass spectrometry (HS-SPME-GCMS). Factors investigated were the mass of equine faeces, type of SPME fibre coating, vial volume and storage conditions.
Results: The resultant method was unique to those developed for other species. Aliquots of 1000 or 2000 mg in 10 ml or 20 ml SPME headspace were optimal. From those tested, the extraction of VOCs should ideally be performed using a divinylbenzene-carboxen-polydimethysiloxane (DVB-CAR-PDMS) SPME fibre. Storage of faeces for up to 12 months at - 80 °C shared a greater percentage of VOCs with a fresh sample than the equivalent stored at - 20 °C.
Conclusions: An optimised method for extracting VOCs from equine faeces using HS-SPME-GCMS has been developed and will act as a standard to enable comparisons between studies. This work has also highlighted storage conditions as an important factor to consider in experimental design for faecal metabolomics studies.
Keywords: Equine; Faeces; GC-MS; SPME; Volatile organic compounds.
Conflict of interest statement
Compliance with ethical standardsRH, DA and CP declare that they have no conflict of interest.Ethical approval was granted by the University of Liverpool ethics committee (ref: VREC279). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Figures
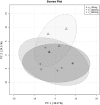
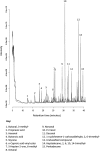
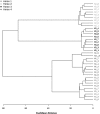

References
-
- Arthur CL, Pawliszyn J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Analytical Chemistry. 1990;62:2145–2148. doi: 10.1021/ac00218a019. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
