Cost-effective downstream processing of recombinantly produced pexiganan peptide and its antimicrobial activity
- PMID: 29368022
- PMCID: PMC5783979
- DOI: 10.1186/s13568-018-0541-3
Cost-effective downstream processing of recombinantly produced pexiganan peptide and its antimicrobial activity
Abstract
Antimicrobial peptides (AMPs) have significant potential as alternatives to classical antibiotics. However, AMPs are currently prepared using processes which are often laborious, expensive and of low-yield, thus hindering their research and application. Large-scale methods for production of AMPs using a cost-effective approach is urgently required. In this study, we report a scalable, chromatography-free downstream processing method for producing an antimicrobial peptide, pexiganan, using recombinant Escherichia coli (E. coli). The four helix bundle structure of the unique carrier protein DAMP4 was used to facilitate a simple and cheap purification process based on a selective thermochemical precipitation. Highly pure fusion protein DAMP4var-pexiganan was obtained at high yield (around 24 mg per 800 mL cell culture with a final cultivation OD600 ~ 2). The purification yield of DAMP4var-pexiganan protein is increased twofold with a 72.9% of the protein recovery in this study as compared to the previous purification processes (Dwyer in Chem Eng Sci 105:12-21, 2014). The antimicrobial peptide pexiganan was released and activated from the fusion protein by a simple acid-cleavage. Isoelectric precipitation was then applied to separate the pexiganan peptide from the DAMP4var protein carrier. The final yield of pure bio-produced pexiganan was 1.6 mg from 800 mL of bacterial cell culture (final cultivation OD600 ~ 2). The minimum bactericidal concentration (MBC) test demonstrated that the bio-produced pexiganan has the same antimicrobial activity as chemically synthesized counterpart. This novel downstream process provides a new strategy for simple and probable economic production of antimicrobial peptides.
Keywords: Antimicrobial peptides; Carrier proteins; Minimum bactericidal concentration; Protein purification; Recombinant E. coli; Selective precipitation.
Figures





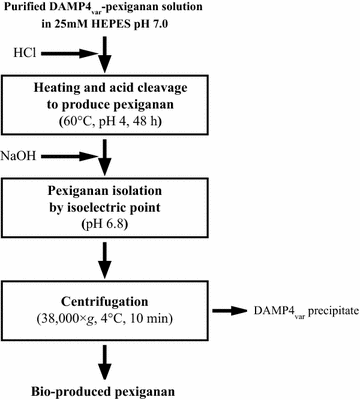

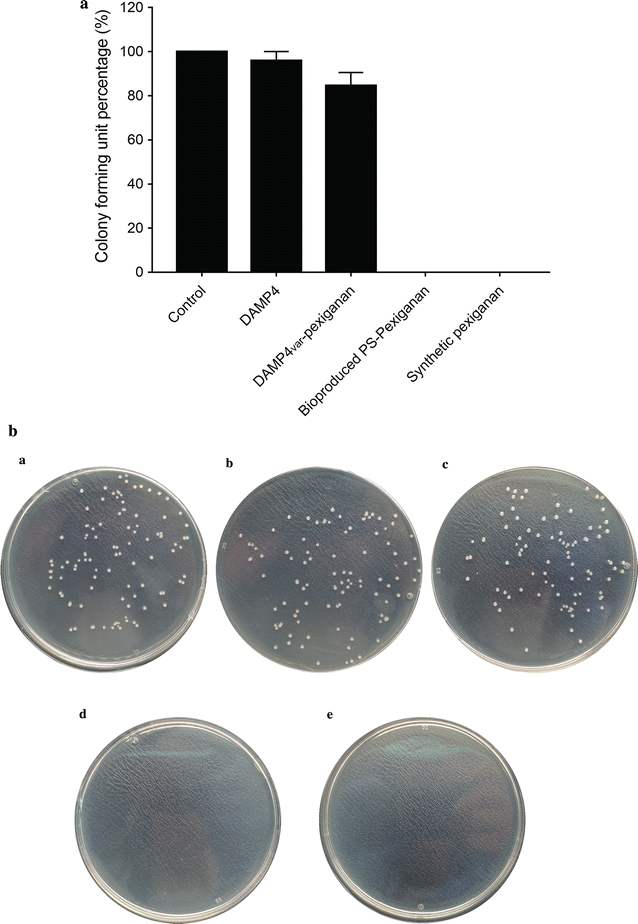

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

