Analysis of novel endosome-to-Golgi retrieval genes reveals a role for PLD3 in regulating endosomal protein sorting and amyloid precursor protein processing
- PMID: 29368044
- PMCID: PMC6003983
- DOI: 10.1007/s00018-018-2752-9
Analysis of novel endosome-to-Golgi retrieval genes reveals a role for PLD3 in regulating endosomal protein sorting and amyloid precursor protein processing
Abstract
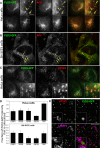
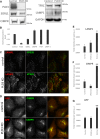
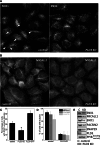
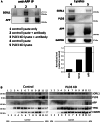
The processing of amyloid precursor protein (APP) to the neurotoxic pro-aggregatory Aβ peptide is controlled by the mechanisms that govern the trafficking and localisation of APP. We hypothesised that genes involved in endosomal protein sorting could play an important role in regulating APP processing and, therefore, analysed ~ 40 novel endosome-to-Golgi retrieval genes previously identified in a genome-wide siRNA screen. We report that phospholipase D3 (PLD3), a type II membrane protein, functions in endosomal protein sorting and plays an important role in regulating APP processing. PLD3 co-localises with APP in endosomes and loss of PLD3 function results in reduced endosomal tubules, impaired trafficking of several membrane proteins and reduced association of sortilin-like 1 with APP.
Keywords: Alzheimer disease; Amyloid precursor protein; Endosome; Phospholipase D; SorL1.
Figures

References
-
- Rajendran L, Schneider A, Schlechtingen G, Weidlich S, Ries J, Braxmeier T, Schwille P, Schulz JB, Schroeder C, Simons M, Jennings G, Knölker HJ, Simons K. Efficient inhibition of the Alzheimer’s disease beta-secretase by membrane targeting. Science. 2008;320(5875):520–523. doi: 10.1126/science.1156609. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

