Assessing the Role of Muscle Protein Breakdown in Response to Nutrition and Exercise in Humans
- PMID: 29368185
- PMCID: PMC5790854
- DOI: 10.1007/s40279-017-0845-5
Assessing the Role of Muscle Protein Breakdown in Response to Nutrition and Exercise in Humans
Abstract
Muscle protein breakdown (MPB) is an important metabolic component of muscle remodeling, adaptation to training, and increasing muscle mass. Degradation of muscle proteins occurs via the integration of three main systems-autophagy and the calpain and ubiquitin-proteasome systems. These systems do not operate independently, and the regulation is complex. Complete degradation of a protein requires some combination of the systems. Determination of MPB in humans is technically challenging, leading to a relative dearth of information. Available information on the dynamic response of MPB primarily comes from stable isotopic methods with expression and activity measures providing complementary information. It seems clear that resistance exercise increases MPB, but not as much as the increase in muscle protein synthesis. Both hyperaminoacidemia and hyperinsulinemia inhibit the post-exercise response of MPB. Available data do not allow a comprehensive examination of the mechanisms behind these responses. Practical nutrition recommendations for interventions to suppress MPB following exercise are often made. However, it is likely that some degree of increased MPB following exercise is an important component for optimal remodeling. At this time, it is not possible to determine the impact of nutrition on any individual muscle protein. Thus, until we can develop and employ better methods to elucidate the role of MPB following exercise and the response to nutrition, recommendations to optimize post exercise nutrition should focus on the response of muscle protein synthesis. The aim of this review is to provide a comprehensive examination of the state of knowledge, including methodological considerations, of the response of MPB to exercise and nutrition in humans.
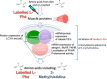
Figures
References
-
- Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–482. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

