A calcium optimum for cytotoxic T lymphocyte and natural killer cell cytotoxicity
- PMID: 29368348
- PMCID: PMC6046087
- DOI: 10.1113/JP274964
A calcium optimum for cytotoxic T lymphocyte and natural killer cell cytotoxicity
Abstract
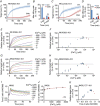
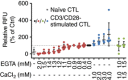
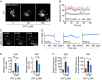
Key points: Cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells are required to eliminate cancer cells. We analysed the Ca2+ dependence of CTL and NK cell cytotoxicity and found that in particular CTLs have a very low optimum of [Ca2+ ]i (between 122 and 334 nm) and [Ca2+ ]o (between 23 and 625 μm) for efficient cancer cell elimination, well below blood plasma Ca2+ levels. As predicted from these results, partial down-regulation of the Ca2+ channel Orai1 in CTLs paradoxically increases perforin-dependent cancer cell killing. Lytic granule release at the immune synapse between CTLs and cancer cells has a Ca2+ optimum compatible with this low Ca2+ optimum for efficient cancer cell killing, whereas the Ca2+ optimum for CTL migration is slightly higher and proliferation increases monotonously with increasing [Ca2+ ]o . We propose that a partial inhibition of Ca2+ signals by specific Orai1 blockers at submaximal concentrations could contribute to tumour elimination.
Abstract: Cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells are required to protect the human body against cancer. Ca2+ is a key metabolic factor for lymphocyte function and cancer homeostasis. We analysed the Ca2+ dependence of CTL and NK cell cytotoxicity against cancer cells and found that CTLs have a bell-shaped Ca2+ dependence with an optimum for cancer cell elimination at rather low [Ca2+ ]o (23-625 μm) and [Ca2+ ]i (122-334 nm). This finding predicts that a partial inhibition of Orai1 should increase (rather than decrease) cytotoxicity of CTLs at [Ca2+ ]o higher than 625 μm. We tested this hypothesis in CTLs and indeed found that partial down-regulation of Orai1 by siRNA increases the efficiency of cancer cell killing. We found two mechanisms that may account for the Ca2+ optimum of cancer cell killing: (1) migration velocity and persistence have a moderate optimum between 500 and 1000 μm [Ca2+ ]o in CTLs, and (2) lytic granule release at the immune synapse between CTLs and cancer cells is increased at 146 μm compared to 3 or 800 μm, compatible with the Ca2+ optimum for cancer cell killing. It has been demonstrated in many cancer cell types that Orai1-dependent Ca2+ signals enhance proliferation. We propose that a decrease of [Ca2+ ]o or partial inhibition of Orai1 activity by selective blockers in the tumour microenvironment could efficiently reduce cancer growth by simultaneously increasing CTL and NK cell cytotoxicity and decreasing cancer cell proliferation.
Keywords: cancer cells; cytotoxic immune cells; killing efficiency.
© 2018 The Authors. The Journal of Physiology © 2018 The Physiological Society.
Figures



References
-
- Becherer U, Medart MR, Schirra C, Krause E, Stevens D & Rettig J (2012). Regulated exocytosis in chromaffin cells and cytotoxic T lymphocytes: how similar are they? Cell Calcium 52, 303–312. - PubMed
-
- Bhat SS, Friedmann KS, Knorck A, Hoxha C, Leidinger P, Backes C, Meese E, Keller A, Rettig J, Hoth M, Qu B & Schwarz EC (2016). Syntaxin 8 is required for efficient lytic granule trafficking in cytotoxic T lymphocytes. Biochim Biophys Acta 1863, 1653–1664. - PubMed
-
- Chaigne‐Delalande B, Li FY, O'Connor GM, Lukacs MJ, Jiang P, Zheng L, Shatzer A, Biancalana M, Pittaluga S, Matthews HF, Jancel TJ, Bleesing JJ, Marsh RA, Kuijpers TW, Nichols KE, Lucas CL, Nagpal S, Mehmet H, Su HC, Cohen JI, Uzel G & Lenardo MJ (2013). Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science 341, 186–191. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

