Study of platelet-rich fibrin combined with rat periodontal ligament stem cells in periodontal tissue regeneration
- PMID: 29368432
- PMCID: PMC5783838
- DOI: 10.1111/jcmm.13461
Study of platelet-rich fibrin combined with rat periodontal ligament stem cells in periodontal tissue regeneration
Abstract
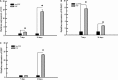
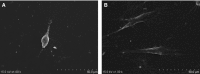
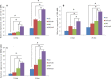
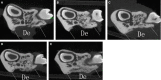
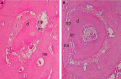
The objective of this study was to investigate the advantages and feasibility of periodontal tissue regeneration using platelet-rich fibrin (PRF) combined with rat periodontal ligament stem cells (PDLSCs) for the first time. We first determined the effect of PRF on rat PDLSCs in vitro. We next conducted an in vivo study, in which a tissue engineering technique was performed to repair periodontal defects in five groups: a blank group, collagen group (implanted collagen membrane), collagen + cells group (implanted collagen membrane and rat PDLSCs), PRF group (implanted PRF membrane) and PRF + cells group (implanted PRF membrane and rat PDLSCs). PRF greatly enhanced cell proliferation, mRNA and protein expression levels of bone sialoprotein (BSP), osteocalcin (OC), and runt-related transcription factor 2 (RUNX2) and activity of alkaline phosphatase (ALP) in vitro. Transplantation of PRF combined with rat PDLSCs resulted in higher expression of osteopontin (Opn), collagen I (COL1A) and RUNX2 at both 12 and 24 days after surgery. Micro-computed tomography and histological analysis showed substantially more new bone formation in the PRF + cells group at 24 days after surgery. Based on these results, we discuss the role of PRF in the proliferation and differentiation of rat PDLSCs and suggest that PRF combined with rat PDLSCs provides a valuable tool for periodontal tissue engineering.
Keywords: periodontal ligament stem cells; periodontal regeneration; platelet-rich fibrin.
© 2017 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures



References
-
- Ivanovski S. Periodontal regeneration. Aust Dent J. 2009; 54: S118–28. - PubMed
-
- Seo BM, Miura M, Gronthos S, et al Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004; 364: 149–55. - PubMed
-
- Tassi SA, Sergio NZ, Misawa MYO, et al Efficacy of stem cells on periodontal regeneration: systematic review of pre‐clinical studies. J Periodontal Res. 2017; 52: 793–812. - PubMed
-
- Kim BS, Mooney DJ. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998; 16: 224–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

