The Virtual Anemia Trial: An Assessment of Model-Based In Silico Clinical Trials of Anemia Treatment Algorithms in Patients With Hemodialysis
- PMID: 29368434
- PMCID: PMC5915606
- DOI: 10.1002/psp4.12276
The Virtual Anemia Trial: An Assessment of Model-Based In Silico Clinical Trials of Anemia Treatment Algorithms in Patients With Hemodialysis
Abstract
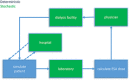
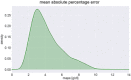
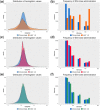
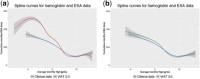
In silico approaches have been proposed as a novel strategy to increase the repertoire of clinical trial designs. Realistic simulations of clinical trials can provide valuable information regarding safety and limitations of treatment protocols and have been shown to assist in the cost-effective planning of clinical studies. In this report, we present a blueprint for the stepwise integration of internal, external, and ecological validity considerations in virtual clinical trials (VCTs). We exemplify this approach in the context of a model-based in silico clinical trial aimed at anemia treatment in patients undergoing hemodialysis (HD). Hemoglobin levels and subsequent anemia treatment were simulated on a per patient level over the course of a year and compared to real-life clinical data of 79,426 patients undergoing HD. The novel strategies presented here, aimed to improve external and ecological validity of a VCT, significantly increased the predictive power of the discussed in silico trial.
© 2018 The Authors CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures
Similar articles
-
2008 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease.Ther Apher Dial. 2010 Jun;14(3):240-75. doi: 10.1111/j.1744-9987.2010.00836.x. Ther Apher Dial. 2010. PMID: 20609178
-
[REIN Report 2011--summary].Nephrol Ther. 2013 Sep;9 Suppl 1:S3-6. doi: 10.1016/S1769-7255(13)70036-1. Nephrol Ther. 2013. PMID: 24119584 French.
-
Performance of a Predictive Model for Long-Term Hemoglobin Response to Darbepoetin and Iron Administration in a Large Cohort of Hemodialysis Patients.PLoS One. 2016 Mar 3;11(3):e0148938. doi: 10.1371/journal.pone.0148938. eCollection 2016. PLoS One. 2016. PMID: 26939055 Free PMC article.
-
Impact of dialysis technique on renal anemia.Contrib Nephrol. 2011;171:261-265. doi: 10.1159/000327341. Epub 2011 May 23. Contrib Nephrol. 2011. PMID: 21625122 Review.
-
Clinical efficacy of recombinant human erythropoietin in hemodialysis patients.Semin Nephrol. 1989 Mar;9(1 Suppl 1):16-21. Semin Nephrol. 1989. PMID: 2648516 Review.
Cited by
-
System-based approaches as prognostic tools for glioblastoma.BMC Cancer. 2019 Nov 12;19(1):1092. doi: 10.1186/s12885-019-6280-2. BMC Cancer. 2019. PMID: 31718568 Free PMC article. Review.
-
Alternative strategies in cardiac preclinical research and new clinical trial formats.Cardiovasc Res. 2022 Feb 21;118(3):746-762. doi: 10.1093/cvr/cvab075. Cardiovasc Res. 2022. PMID: 33693475 Free PMC article. Review.
-
Festschrift in honor of Dr. Jeffrey Hymes.Front Nephrol. 2025 May 28;5:1585713. doi: 10.3389/fneph.2025.1585713. eCollection 2025. Front Nephrol. 2025. PMID: 40502663 Free PMC article.
-
In silico trial for the assessment of givinostat dose adjustment rules based on the management of key hematological parameters in polycythemia vera patients.CPT Pharmacometrics Syst Pharmacol. 2024 Mar;13(3):359-373. doi: 10.1002/psp4.13087. Epub 2024 Feb 7. CPT Pharmacometrics Syst Pharmacol. 2024. PMID: 38327117 Free PMC article.
-
Mechanisms of hemoglobin cycling in anemia patients treated with erythropoiesis-stimulating agents.PLoS Comput Biol. 2023 Jan 24;19(1):e1010850. doi: 10.1371/journal.pcbi.1010850. eCollection 2023 Jan. PLoS Comput Biol. 2023. PMID: 36693034 Free PMC article.
References
-
- Hoos, W.A. , James, P.M. , Rahib, L. , Talley, A.W. , Fleshman, J.M. & Matrisian, L.M. Pancreatic cancer clinical trials and accrual in the United States. J. Clin. Oncol. 31, 3432–3438 (2013). - PubMed
-
- Sergeyeva, O. et al Challenges to enrollment and randomization of the Frequent Hemodialysis Network (FHN) daily trial. J. Nephrol. 25, 302–309 (2012). - PubMed
-
- Bhatt, D.L. & Mehta, C. Adaptive designs for clinical trials. N. Engl. J. Med. 375, 65–74 (2016). - PubMed
-
- Clermont, G. , Bartels, J. , Kumar, R. , Constantine, G. , Vodovotz, Y. & Chow, C. In silico design of clinical trials: a method coming of age. Crit. Care Med. 32, 2061–2070 (2004). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

