Lactobacillus reuteri strains protect epithelial barrier integrity of IPEC-J2 monolayers from the detrimental effect of enterotoxigenic Escherichia coli
- PMID: 29368445
- PMCID: PMC5789714
- DOI: 10.14814/phy2.13514
Lactobacillus reuteri strains protect epithelial barrier integrity of IPEC-J2 monolayers from the detrimental effect of enterotoxigenic Escherichia coli
Abstract
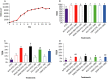
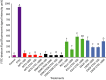
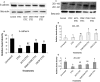
Lactobacillus reuteri is an inhabitant of the gastrointestinal (GI) tract of mammals and birds and several strains of this species are known to be effective probiotics. The mechanisms by which L. reuteri confers its health-promoting effects are far from being fully understood, but protection of the mucosal barrier is thought to be important. Leaky gut is a state of abnormal intestinal permeability with implications for the pathophysiology of various gastrointestinal disorders. Enterotoxigenic Escherichia coli (ETEC) can invade the intestinal mucosa and induce changes in barrier function by producing enterotoxin or by direct invasion of the intestinal epithelium. Our hypothesis was that L. reuteri can protect the mucosal barrier, and the goal of the study was to challenge this hypothesis by monitoring the protective effect of L. reuteri strains on epithelial dysfunction caused by ETEC. Using an infection model based on the porcine intestinal cell line IPEC-J2, it was demonstrated that pretreatment of the cells with human-derived L. reuteri strains (ATCC PTA 6475, DSM 17938 and 1563F) and a rat strain (R2LC) reduced the detrimental effect of ETEC in a dose-dependent manner, as monitored by permeability of FITC-dextran and transepithelial electrical resistance (TEER). Moreover, the results revealed that ETEC upregulated proinflammatory cytokines IL-6 and TNFα and decreased expression of the shorter isoform of ZO-1 (187 kDa) and E-cadherin. In contrast, pretreatment with L. reuteri DSM 17938 and 1563F downregulated expression of IL-6 and TNFα, and led to an increase in production of the longer isoform of ZO-1 (195 kDa) and maintained E-cadherin expression. Interestingly, expression of ZO-1 (187 kDa) was preserved only when the infected cells were pretreated with strain 1563F. These findings demonstrate that L. reuteri strains exert a protective effect against ETEC-induced mucosal integrity disruption.
Keywords: Lactobacillus reuteri; Enterotoxigenic Escherichia coli (ETEC); IPEC-J2; mucosal integrity.
© 2018 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures

References
-
- Ahrné, S. , Molin G., and Axelsson L.. 1992. Transformation of Lactobacillus reuteri with electroporation: studies on the erythromycin resistance plasmid pLUL631. Curr. Microbiol. 24:199–205.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

