Chromatin modifications in metabolic disease: Potential mediators of long-term disease risk
- PMID: 29369528
- PMCID: PMC6002879
- DOI: 10.1002/wsbm.1416
Chromatin modifications in metabolic disease: Potential mediators of long-term disease risk
Abstract
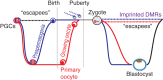
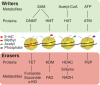
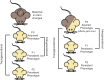
Metabolic diseases such as obesity and diabetes are complex diseases resulting from multiple genetic and environmental factors, such as diet and activity levels. These factors are well known contributors to the development of metabolic diseases. One manner by which environmental factors can influence metabolic disease progression is through modifications to chromatin. These modifications can lead to altered gene regulatory programs, which alters disease risk. Furthermore, there is evidence that parents exposed to environmental factors can influence the metabolic health of offspring, especially if exposures are during intrauterine growth periods. In this review, we outline the evidence that chromatin modifications are associated with metabolic diseases, including diabetes and obesity. We also consider evidence that these chromatin modifications can lead to long-term disease risk and contribute to disease risk for future generations. This article is categorized under: Biological Mechanisms > Metabolism Developmental Biology > Developmental Processes in Health and Disease Physiology > Organismal Responses to Environment.
© 2018 The Authors. WIREs Systems Biology and Medicine published by Wiley Periodicals, Inc.
Figures



References
-
- Ahima, R. S. (2009). Connecting obesity, aging and diabetes. Nature Medicine, 15(9), 996–997. https://doi.org/10.1038/nm0909-996 - DOI - PubMed
-
- Anderson, J. W. , Konz, E. C. , Frederich, R. C. , & Wood, C. L. (2001). Long‐term weight‐loss maintenance: A meta‐analysis of US studies. American Journal of Clinical Nutrition, 74(5), 579–584. - PubMed
-
- Bannister, A. J. , & Kouzarides, T. (2011). Regulation of chromatin by histone modifications. Cell Research, 21(3), 381–395. https://doi.org/10.1038/cr.2011.22 - DOI - PMC - PubMed
-
- Bestor, T. H. (2000). The DNA methyltransferases of mammals. Human Molecular Genetics, 9(16), 2395–2402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

