Evaluation of an individualized dose titration regimen of patiromer to prevent hyperkalaemia in patients with heart failure and chronic kidney disease
- PMID: 29369537
- PMCID: PMC5933966
- DOI: 10.1002/ehf2.12265
Evaluation of an individualized dose titration regimen of patiromer to prevent hyperkalaemia in patients with heart failure and chronic kidney disease
Abstract
Aims: Hyperkalaemia risk precludes optimal renin-angiotensin-aldosterone system inhibitor use in patients with heart failure (HF), particularly those with chronic kidney disease (CKD). Patiromer is a sodium-free, non-absorbed potassium (K+ )-binding polymer approved for the treatment of hyperkalaemia. In PEARL-HF, patiromer 25.2 g (fixed dose) prevented hyperkalaemia in HF patients with or without CKD initiating spironolactone. The current study evaluated the effectiveness of a lower starting dose of patiromer (16.4 g/day) followed by individualized titration in preventing hyperkalaemia and hypokalaemia when initiating spironolactone.
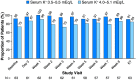
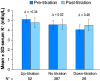
Methods and results: This open-label 8-week study enrolled 63 patients with CKD, serum K+ 4.3-5.1 mEq/L, and chronic HF, who, based on investigator opinion, should receive spironolactone. Eligible patients started spironolactone 25 mg/day and patiromer 16.8 g/day (divided into two doses), with patiromer titrated to maintain serum K+ 4.0-5.1 mEq/L. Mean (standard deviation) serum K+ was 4.78 (0.51) mEq/L at baseline; weekly values were 4.48-4.70 mEq/L during treatment. Serum K+ of 3.5-5.5 mEq/L at the end of study treatment (primary endpoint) was achieved by 57 (90.5%) patients; 53 (84.1%) had serum K+ 4.0-5.1 mEq/L. One patient (1.6%) developed hypokalaemia, and two patients (3.2%) developed hypomagnesaemia. Spironolactone was increased to 50 mg/day in all patients; 43 (68%) patients required one or more patiromer dose titration. Adverse events (AEs) occurred in 36 (57.1%) patients, with a low rate of discontinuations [four (6.3%) patients]. The most common AE was mild to moderate abdominal discomfort [four (6.3%) patients].
Conclusions: In this open-label study, patiromer 16.8 g/day followed by individualized titration maintained serum K+ within the target range in the majority of patients with HF and CKD, all of whom were uptitrated to spironolactone 50 mg/day, patiromer was well tolerated, with a low incidence of hyperkalaemia, hypokalaemia, and hypomagnesaemia.
Trial registration: ClinicalTrials.gov NCT01130597.
Keywords: Chronic kidney disease; Heart failure; Mineralocorticoid receptor antagonist; Patiromer; Potassium-binding polymer.
© 2018 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of the European Society of Cardiology.
Figures
References
-
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341: 709–717. - PubMed
-
- Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleinman J, Gatlin M, Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators . Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348: 1309–1321. - PubMed
-
- Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, EMPHASIS‐HF Study Group . Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11–21.
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70: 776–803. - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PE, Stevenson LW, Westlake C. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2016; 27: 1476–1488. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous