Mitochondria are physiologically maintained at close to 50 °C
- PMID: 29370167
- PMCID: PMC5784887
- DOI: 10.1371/journal.pbio.2003992
Mitochondria are physiologically maintained at close to 50 °C
Abstract
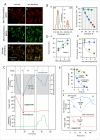
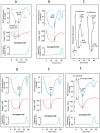
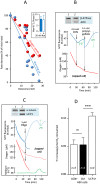
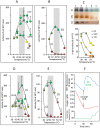
In endothermic species, heat released as a product of metabolism ensures stable internal temperature throughout the organism, despite varying environmental conditions. Mitochondria are major actors in this thermogenic process. Part of the energy released by the oxidation of respiratory substrates drives ATP synthesis and metabolite transport, but a substantial proportion is released as heat. Using a temperature-sensitive fluorescent probe targeted to mitochondria, we measured mitochondrial temperature in situ under different physiological conditions. At a constant external temperature of 38 °C, mitochondria were more than 10 °C warmer when the respiratory chain (RC) was fully functional, both in human embryonic kidney (HEK) 293 cells and primary skin fibroblasts. This differential was abolished in cells depleted of mitochondrial DNA or treated with respiratory inhibitors but preserved or enhanced by expressing thermogenic enzymes, such as the alternative oxidase or the uncoupling protein 1. The activity of various RC enzymes was maximal at or slightly above 50 °C. In view of their potential consequences, these observations need to be further validated and explored by independent methods. Our study prompts a critical re-examination of the literature on mitochondria.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Arai S, Suzuki M, Park SJ, Yoo JS, Wang L, et al. (2015) Mitochondria-targeted fluorescent thermometer monitors intracellular temperature gradient. Chem Commun (Camb) 51: 8044–8047. - PubMed
-
- Abad MF, Di Benedetto G, Magalhaes PJ, Filippin L, Pozzan T (2004) Mitochondrial pH monitored by a new engineered green fluorescent protein mutant. J Biol Chem 279: 11521–11529. doi: 10.1074/jbc.M306766200 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

