Early life vitamin D depletion alters the postnatal response to skeletal loading in growing and mature bone
- PMID: 29370213
- PMCID: PMC5784894
- DOI: 10.1371/journal.pone.0190675
Early life vitamin D depletion alters the postnatal response to skeletal loading in growing and mature bone
Abstract
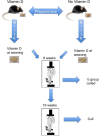
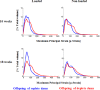
There is increasing evidence of persistent effects of early life vitamin D exposure on later skeletal health; linking low levels in early life to smaller bone size in childhood as well as increased fracture risk later in adulthood, independently of later vitamin D status. A major determinant of bone mass acquisition across all ages is mechanical loading. We tested the hypothesis in an animal model system that early life vitamin D depletion results in abrogation of the response to mechanical loading, with consequent reduction in bone size, mass and strength during both childhood and adulthood. A murine model was created in which pregnant dams were either vitamin D deficient or replete, and their offspring moved to a vitamin D replete diet at weaning. Tibias of the offspring were mechanically loaded and bone structure, extrinsic strength and growth measured both during growth and after skeletal maturity. Offspring of vitamin D deplete mice demonstrated lower bone mass in the non loaded limb and reduced bone mass accrual in response to loading in both the growing skeleton and after skeletal maturity. Early life vitamin D depletion led to reduced bone strength and altered bone biomechanical properties. These findings suggest early life vitamin D status may, in part, determine the propensity to osteoporosis and fracture that blights later life in many individuals.
Conflict of interest statement
Figures




References
-
- Bonjour JP, Chevalley T. Pubertal timing, bone acquisition, and risk of fracture throughout life. Endocrine reviews. 2014;35(5):820–47. doi: 10.1210/er.2014-1007 . - DOI - PubMed
-
- Hendrickx G, Boudin E, Van Hul W. A look behind the scenes: The risk and pathogenesis of primary osteoporosis. Nature Reviews Rheumatology. 2015;11(8):462–74. doi: 10.1038/nrrheum.2015.48 - DOI - PubMed
-
- Kimball S, Fuleihan Gel H, Vieth R. Vitamin D: a growing perspective. Critical reviews in clinical laboratory sciences. 2008;45(4):339–414. doi: 10.1080/10408360802165295 . - DOI - PubMed
-
- Ioannou C, Javaid MK, Mahon P, Yaqub MK, Harvey NC, Godfrey KM, et al. The effect of maternal vitamin D concentration on fetal bone. Journal of Clinical Endocrinology and Metabolism. 2012;97(11):E2070–E7. doi: 10.1210/jc.2012-2538 - DOI - PMC - PubMed
-
- Mahon P, Harvey N, Crozier S, Inskip H, Robinson S, Arden N, et al. Low maternal vitamin D status and fetal bone development: Cohort study. Journal of Bone and Mineral Research. 2010;25(1):14–9. doi: 10.1359/jbmr.090701 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

