Risk factors for death, stroke, and bleeding in 28,628 patients from the GARFIELD-AF registry: Rationale for comprehensive management of atrial fibrillation
- PMID: 29370229
- PMCID: PMC5784935
- DOI: 10.1371/journal.pone.0191592
Risk factors for death, stroke, and bleeding in 28,628 patients from the GARFIELD-AF registry: Rationale for comprehensive management of atrial fibrillation
Abstract
Background: The factors influencing three major outcomes-death, stroke/systemic embolism (SE), and major bleeding-have not been investigated in a large international cohort of unselected patients with newly diagnosed atrial fibrillation (AF).
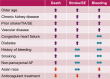
Methods and results: In 28,628 patients prospectively enrolled in the GARFIELD-AF registry with 2-year follow-up, we aimed at analysing: (1) the variables influencing outcomes; (2) the extent of implementation of guideline-recommended therapies in comorbidities that strongly affect outcomes. Median (IQR) age was 71.0 (63.0 to 78.0) years, 44.4% of patients were female, median (IQR) CHA2DS2-VASc score was 3.0 (2.0 to 4.0); 63.3% of patients were on anticoagulants (ACs) with or without antiplatelet (AP) therapy, 24.5% AP monotherapy, and 12.2% no antithrombotic therapy. At 2 years, rates (95% CI) of death, stroke/SE, and major bleeding were 3.84 (3.68; 4.02), 1.27 (1.18; 1.38), and 0.71 (0.64; 0.79) per 100 person-years. Age, history of stroke/SE, vascular disease (VascD), and chronic kidney disease (CKD) were associated with the risks of all three outcomes. Congestive heart failure (CHF) was associated with the risks of death and stroke/SE. Smoking, non-paroxysmal forms of AF, and history of bleeding were associated with the risk of death, female sex and heavy drinking with the risk of stroke/SE. Asian race was associated with lower risks of death and major bleeding versus other races. AC treatment was associated with 30% and 28% lower risks of death and stroke/SE, respectively, compared with no AC treatment. Rates of prescription of guideline-recommended drugs were suboptimal in patients with CHF, VascD, or CKD.
Conclusions: Our data show that several variables are associated with the risk of one or more outcomes, in terms of death, stroke/SE, and major bleeding. Comprehensive management of AF should encompass, besides anticoagulation, improved implementation of guideline-recommended therapies for comorbidities strongly associated with outcomes, namely CHF, VascD, and CKD.
Trial registration: ClinicalTrials.gov NCT01090362.
Conflict of interest statement
Figures

References
-
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr., et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071–104. doi: 10.1161/CIR.0000000000000040 - DOI - PubMed
-
- Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–962. doi: 10.1093/eurheartj/ehw210 - DOI - PubMed
-
- Bassand JP, Accetta G, Camm AJ, Cools F, Fitzmaurice DA, Fox KA, et al. Two-year outcomes of patients with newly diagnosed atrial fibrillation: results from GARFIELD-AF. Eur Heart J. 2016;37(38):2882–9. doi: 10.1093/eurheartj/ehw233 - DOI - PMC - PubMed
-
- Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol. 2016;23(11):Np1–np96. doi: 10.1177/2047487316653709 - DOI - PubMed
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. doi: 10.1093/eurheartj/ehw128 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

