Effects of the sodium-glucose co-transporter 2 inhibitor dapagliflozin in patients with type 2 diabetes and Stages 3b-4 chronic kidney disease
- PMID: 29370424
- PMCID: PMC6212718
- DOI: 10.1093/ndt/gfx350
Effects of the sodium-glucose co-transporter 2 inhibitor dapagliflozin in patients with type 2 diabetes and Stages 3b-4 chronic kidney disease
Erratum in
-
Effects of the sodium-glucose co-transporter 2 inhibitor dapagliflozin in patients with type 2 diabetes and Stages 3b-4 chronic kidney disease.Nephrol Dial Transplant. 2018 Jul 1;33(7):1280. doi: 10.1093/ndt/gfy135. Nephrol Dial Transplant. 2018. PMID: 29762740 Free PMC article. No abstract available.
Abstract
Background: The sodium-glucose co-transporter 2 inhibitor dapagliflozin decreases haemoglobin A1c (HbA1c), body weight, blood pressure (BP) and urinary albumin:creatinine ratio (UACR) in patients with type 2 diabetes. The efficacy and safety of this drug have not been properly defined in patients with type 2 diabetes and Stages 3b-4 chronic kidney disease (CKD).
Methods: In a pooled analysis of 11 phase 3 randomized controlled clinical trials, we determined least square mean changes in HbA1c, body weight, BP, estimated glomerular filtration rate (eGFR) and UACR over 102 weeks in patients with type 2 diabetes and an eGFR between 12 to less than 45 mL/min/1.73 m2 receiving placebo (n = 69) or dapagliflozin 5 or 10 mg (n = 151). Effects on UACR were determined in a subgroup of patients with baseline UACR ≥30 mg/g (n = 136).
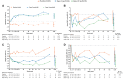
Results: Placebo-corrected changes in HbA1c with dapagliflozin 5 and 10 mg were 0.03% [95% confidence interval (CI) -0.3-0.3] and 0.03% (95% CI -0.2-0.3) during the overall 102-week period. Dapagliflozin 5 and 10 mg compared with placebo reduced UACR by - 47.1% (95% CI -64.8 to - 20.6) and -38.4% (95% CI -57.6 to - 10.3), respectively. Additionally, dapagliflozin 5 and 10 mg compared with placebo reduced BP and body weight. eGFR increased with placebo during the first 4 weeks but did not change with dapagliflozin. There were no between-group differences in eGFR at the end of follow-up. Adverse events associated with renal function occurred more frequently in the dapagliflozin 10-mg group. These events were mainly asymptomatic increases in serum creatinine.
Conclusions: Dapagliflozin did not decrease HbA1c in patients with type 2 diabetes and Stages 3b-4 CKD, but decreased UACR, BP and body weight to a clinically meaningful extent. These results support a large outcome trial in this population to confirm long-term safety and efficacy in reducing adverse clinical endpoints.
Figures

References
-
- Heerspink HJ, Perkins BA, Fitchett DH. et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016; 134: 752–772 - PubMed
-
- Wanner C, Inzucchi SE, Lachin JM. et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

